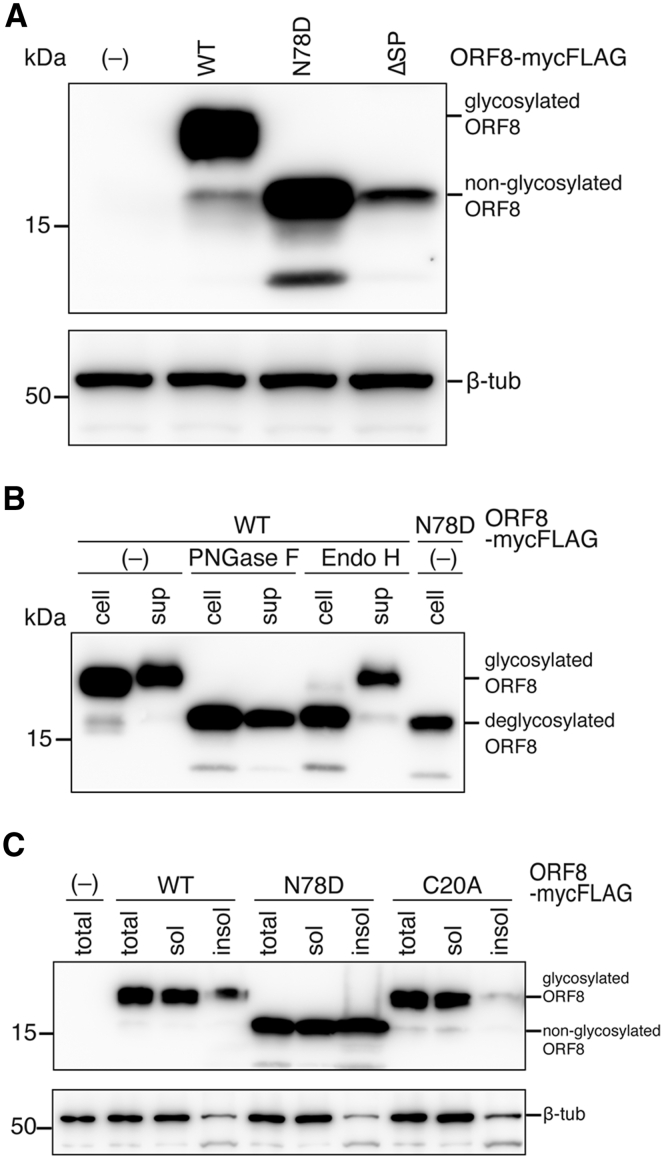
Figure 2.
Characterization of the N-linked glycosylation of the SARS-CoV-2 ORF8 protein.A, briefly, 293T cells were transfected with the wild-type (WT) or mutant ORF8-mycFLAG expression plasmid. Cell lysates were denatured under reducing conditions with 2-ME and then visualized by immunoblotting with an anti-FLAG mAb and anti-β-tub antibody. The ORF8 mutant deficient for the signal sequence (ΔSP) was used as a negative control. B, the Endo H and PNGase F sensitivities of the ORF8 proteins were assessed. The WT and N78D mutant were pulled down with anti-FLAG mAb beads from the cell lysate (cell) and culture supernatant (sup). The IP factions were untreated (–), or incubated with either PNGase F or Endo H glycosidase. The digested samples were subjected to immunoblot analysis using an anti-FLAG mAb. C, the total fraction (total), soluble fraction (sol), and insoluble fraction (insol) were analyzed by Western blot using anti-FLAG mAb and anti-β-tub antibody. 2-ME, 2-mercaptoethanol; ORF8, open reading frame 8; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.