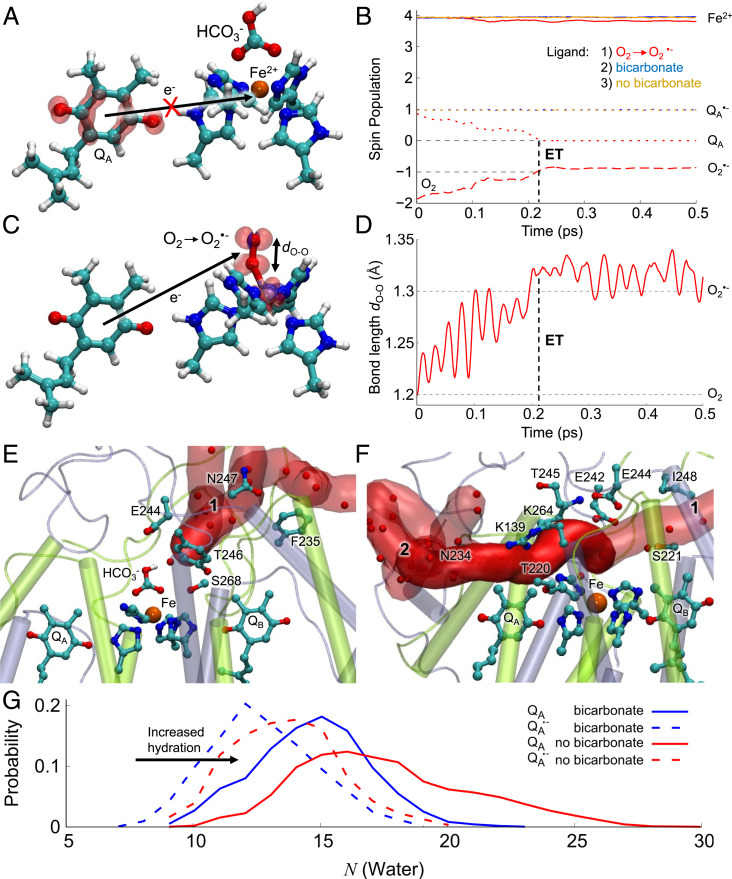
Fig. 4.
O2 reduction mechanism, bicarbonate binding, and accessibility of the nonheme Fe site of PSII. Structure and spin densities from DFT models of the (A) HCO3−-bound and (C) O2-bound forms of the nonheme iron (the state without HCO3− is shown in SI Appendix, Fig. S13F). The figure shows the spin density difference (red/blue sphere ±0.001e for alpha/beta spin) after adding an electron into the system, with only the QA and iron/histidine residues shown for clarity (SI Appendix, Fig. S13). (B) Spin population during the QM/MM MD simulations for HCO3−-bound (in blue) and without HCO3−-bound (in orange) and the O2-bound forms (in red) on the Fe2+ (solid lines), QA (dotted lines), and O2 (dashed line). (D) The O-O bond length during electron transfer from QA. (E and F) Snapshot at 150 ns of the water-filled tunnels formed around the QA site during MD simulations in (E) with the HCO3−-bound form and (F) without the HCO3−. (G) Histogram of water molecules in tunnels 1 and 2 connecting to the iron from the bulk solvent. The bicarbonate blocks water entry to the nonheme iron site.