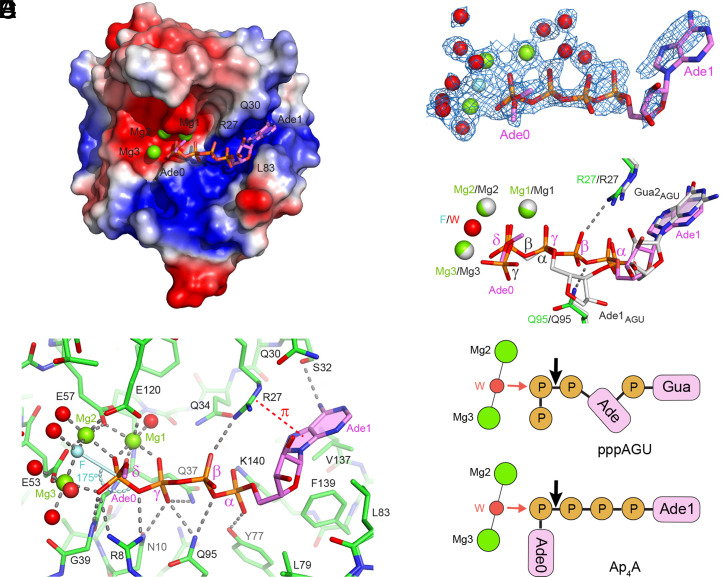
Fig. 5.
Structure of RppH bound to Ap4A. (A) Overall structure of the complex, with RppH in electrostatic surface representation and Ap4A in violet sticks. Mg2+ ions are shown as green spheres. Note the alternative conformations of Ade0 as represented by the location of its C5′ carbon atom. (B) Details of Ap4A binding. The four phosphates of Ap4A are labeled with Greek letters. Green spheres, Mg2+. Cyan sphere (F), fluoride. Red spheres, water molecules that coordinate Mg2+or fluoride. Cyan stick, direction of in-line attack on the δ-phosphate. Curved cyan dashed line, angle of in-line attack by fluoride on the δ-phosphorus atom to displace the bridging oxygen atom. Gray dashed lines, hydrogen bonds and coordination bonds. Red dashed line, cation-π interaction. Only one of the possible locations of the Ade0 C5′ carbon atom is depicted. Note that the spheres are not intended to represent the actual sizes of ions and water molecules. (C) Refined 2Fo-Fc electron density map (1 σ level, blue mesh) shown with the refined model of Ap4A and bound Mg2+ ions. (D) View of the complexes formed by RppH with Ap4A (green and violet) and the triphosphorylated RNA oligonucleotide pppAGU (gray) after all-atom superposition of the corresponding structures [PDB identifiers: 7SP3 (current work) and 4S2Y (16)]. The substrates, magnesium ions, and respective nucleophiles (F, fluoride; W, water) are shown along with the RppH residues (R27 and Q95) that form hydrogen bonds (dashed lines) with the β-phosphate of Ap4A. (E) Schematic representation of substrates bound to RppH, vertically aligned according to the structural superposition in D. (Top) pppAGU; (Bottom) Ap4A. Mg2+ ions (green circles) coordinate (thin black lines) a nucleophilic water molecule (W, red circle). Phosphates (P) are depicted as brown circles and nucleosides as magenta rectangles. Red and black arrows represent in-line nucleophilic attack and the site of phosphoanhydride cleavage, respectively. In the bottom schematic, the fluoride ion used to trap the crystallized enzyme-substrate complex in a catalytically active conformation is replaced by a water molecule normally present there.