Figure 4.
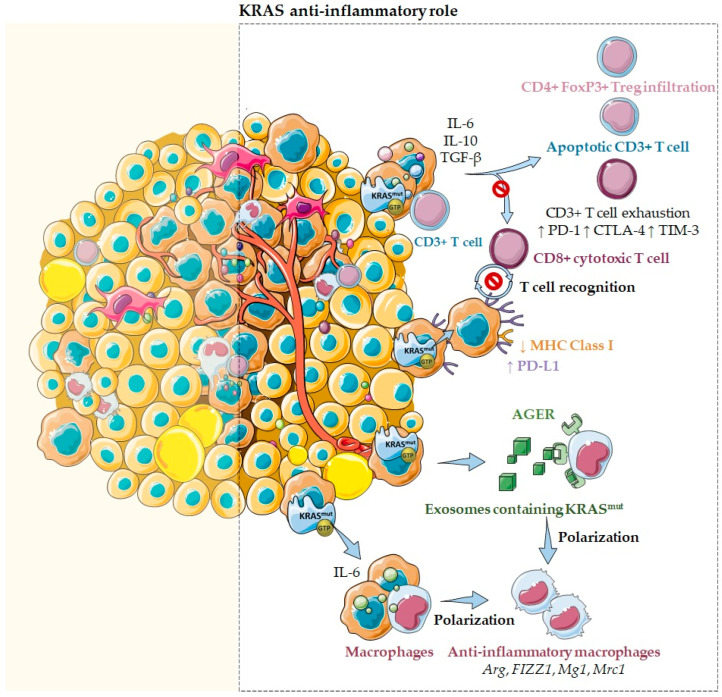
KRAS as an anti-inflammatory tumor microenvironment modulator. Several reports emphasize that KRAS mutations may sustain an anti-inflammatory microenvironment through the secretion of several inflammatory chemokines and cytokines, such as TGF-β, IL-10, and IL-6. In fact, cells harboring KRASG12D mutations secrete high levels of these anti-inflammatory mediators that inhibit T cell activation, suppress cytotoxic CD8+ T cell-mediated tumor killing, and convert pro-inflammatory CD4+ T cells to anti-inflammatory Tregs. Moreover, KRAS mutations were also described to induce the downregulation of MHC class I molecules and the upregulation of PD-L1, reducing the ability of CD8+ cytotoxic T cells to recognize and kill cancer cells. Additionally, KRAS mutations may drive an anti-inflammatory and pro-tumor immune suppressive microenvironment mediated through IL-6 secretion. Notably, when IL-6 was blocked, a reduction of anti-inflammatory macrophage gene expression, such as Arg, FIZZ1, Mg1, and Mrc1, and a reduction of the immunosuppressive cytokines TGF-β and IL-10 were observed. Moreover, it has also been described that IL-6 induces higher levels of T cell exhaustion markers, such as PD-1, CTLA-4, and TIM-3. Furthermore, KRAS mutations effects can also be mediated through exosomes containing KRASG12D. These exosomes can be taken via an AGER-dependent mechanism—a multiligand receptor—by macrophages, modulating their differentiation into a pro-tumor/anti-inflammatory phenotype.