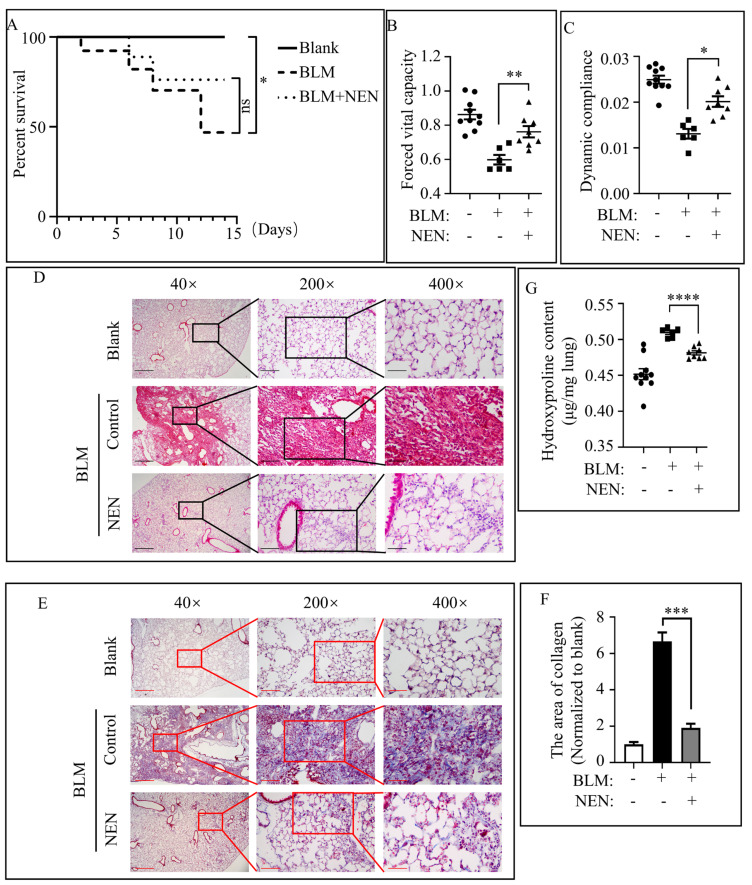
Figure 1.
NEN attenuated IPF in BLM-induced mice. To evaluate the therapeutic effect of NEN in a preclinical model of pulmonary fibrosis, C57BL-6J mice were challenged with bleomycin (BLM) or a 0.9% NaCl solution as a vehicle control (blank) at 2.5 mg/kg body weight; the agents were administered with a liquid lung administration nebulizer. Beginning on day 7 post drug (vehicle) delivery, BLM-challenged mice received NEN (20 mg/kg) or vehicle treatment (i.g.) daily for 2 weeks. (A) The survival rate of each group of mice was counted (n = 10 in the blank group, n = 6 in the BLM group and n = 8 in the NEN group). Pulmonary function was evaluated by assessing parameters including (B) forced vital capacity (FVC) and (C) dynamic compliance (Cdyn) to compare the different treatments. Lung sections were stained with H and E (D) or Masson trichrome (E) to assess collagen accumulation (representative image, magnification 40×, bar = 500 µm, 200×, bar = 100 µm, 400×, bar = 50 µm), and Masson trichrome staining (magnification 40×) was quantified (F) by ImageJ software with comparison to the blank group. (G) The hydroxyproline content in lung tissues among the different groups was analysed and quantified. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.