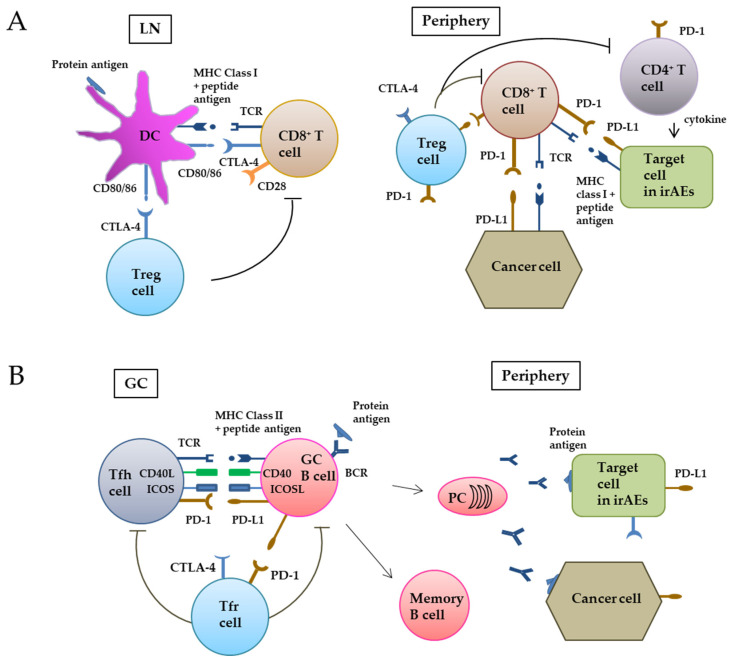
Figure 1.
CTLA-4 and PD-1/PD-L1 in cellular (A) and humoral (B) tumor immunity. (A) In the CD8+ T cell-based pathway, when tumor antigens are released into the tumor microenvironment from dying tumor cells, DCs take up the protein antigens and decomposes them to peptide antigens. In regional lymph nodes, the peptide antigens are cross-presented via MHC class I on the cell surface and recognized by the TCR of CD8+ T cells. In addition, the co-stimulatory binding of CD80/86 of DCs with CD28 of T cells is required for naïve CD8+ T cells to differentiate into cytotoxic CD8+ T cells. Activated CD8+ T cells express the co-inhibitory molecule CTLA-4 to prevent excess activation of CD8+ T cells. CD8+ T cells move to a peripheral tumor site and recognize tumor peptide antigens presented via MHC class I and exert antitumor activity. However, PD-I on CD8+ T cells binds PD-L1 on tumor cells and suppresses the activity of CD8+ T cells. Treg cells locally suppress the activity of CD8+ T cells and CD4+ helper cells. (B) The germinal center (GC) plays an important role in the proliferation and differentiation of B cells. Tfh cells physically bind to GC B cells by co-stimulatory/co-inhibitory pairs such as CD28–CD80/86, CD40L–CD40, ICOS–ICOSL, PD-1, and PD-L1. BCR expressed on B cells detects the protein antigens, and take them into the cells. Peptide antigens processed in B cells are then presented via MHC class II and recognized by Tfh cells. Interaction between Tfh and B cells promotes the differentiation of GC B cells into plasma cells and memory B cells. Tfr cells suppress Tfh and B cells. LN, lymph node; Treg, regulatory T; TCR, T cell receptor; irAEs, immune-related adverse events; GC, germinal center; Tfh, T follicular helper; Tfr, T follicular regulatory; PC, plasma cell; BCR, B cell receptor.