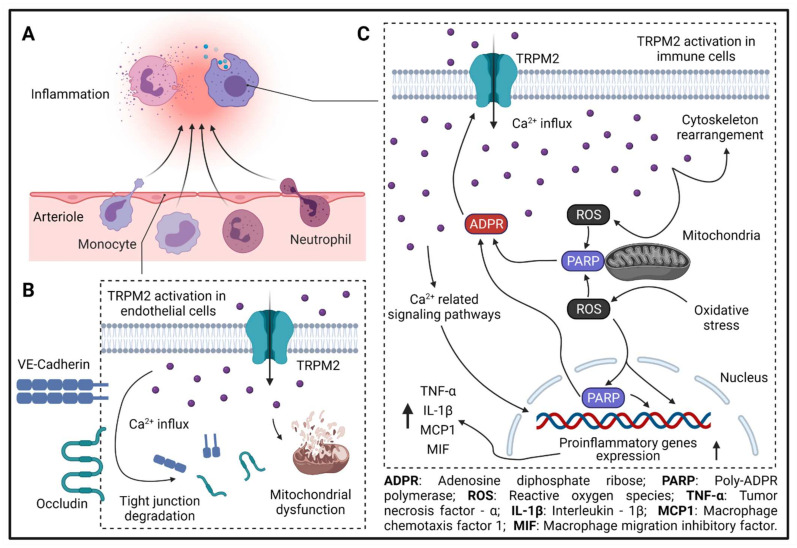
Figure 3.
TRPM2 in inflammation. (A) Leukocyte extravasation during inflammation. (B) TRPM2-mediated Ca2+ influx leads to tight-junction molecule degradation (VE-cadherin and occludin) and mitochondrial dysfunction in endothelial cells. (C) TRPM2-mediated Ca2+ influx is needed for immune cell migration and activation. During inflammation, ROS production in mitochondria is increased, which activates PARP in mitochondria or in the nucleus and enhances the production of ADPR. Increased ADPR potentiates TRPM2-mediated Ca2+ influx, which further increases the production of ROS in mitochondria, leading to the formation of a feed-forward vicious cycle. ROS-, PARP-, and Ca2+ -related signaling pathways increase the expression of proinflammatory genes, such as TNF-α, IL-1β, MCP1, and MIF. Moreover, TRPM2-mediated Ca2+ influx promotes cytoskeleton rearrangement and immune cell migration.