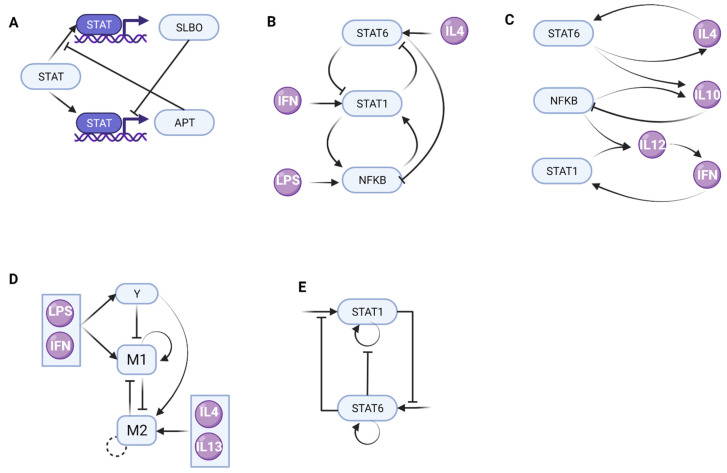
Figure 2.
Models showing multistability of signaling pathways involved in macrophage transitions: (A) The inhibition of STAT activity by APT and the cross-repression of APT and SLBO conforms to a molecular switch that determines if an epithelial cell becomes motile or remains stationary. (B) The mutual inhibition and mutual activation of STAT6, STAT1, and NFKB creates saddle-node bifurcations in macrophage polarization. (C) An agent-based model describes macrophage population behavior in the context of cytokine production to influence bistability switches and demonstrates that a dynamic bifurcation is a crucial built-in mechanism of macrophage activation. (D) A minimal regulatory model shows that a mutual inhibition motif is not by itself sufficient; an incoherent feedforward mechanism of M1 activation as well as both inhibition and activation of M2 by M1 are required for bistability. Y represents feedback inhibition mechanisms. (E) A simple model of macrophage polarization tracking STAT1 and STAT6 activation levels as proxies for M1 and M2 transitions, including self-stimulation and a mutual-inhibition to demonstrate that both external cues and intrinsic pathways are equally important for the emergence of bistability. Figure created with BioRender.com (accessed on 22 December 2021).