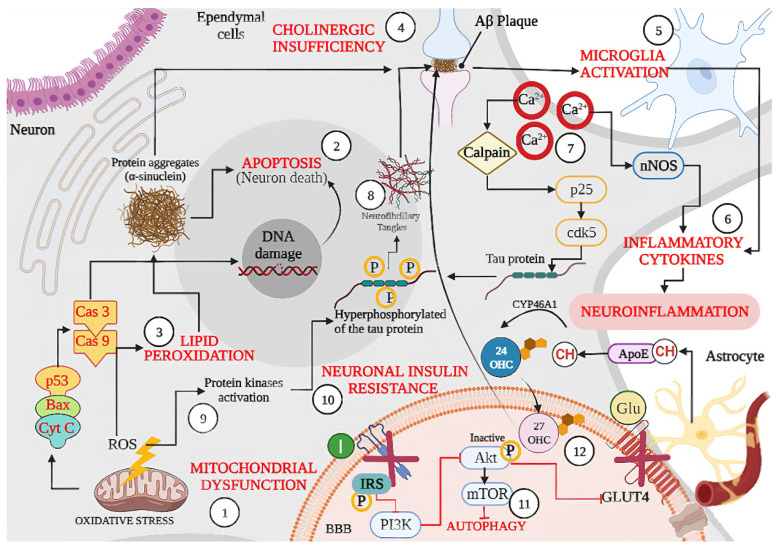
Figure 2.
Schematic representation of shared physiopathological hallmarks in neurodegenerative diseases (NDs): (1) Mitochondrial dysfunction due to oxidative stress, aging, or because of genetic or environmental factors damage, resulting in the excessive production of ROS, which can activate p53 and the Bax (apoptotic regulator) translocation that allows the release of cytochrome C (Cyt C) leading the (Cas 9) and caspase 3 (Cas3) activation, resulting in DNA damage and cell death or (2) Apoptosis. Likewise, excessive ROS production also leads to oxidative stress and (3) Lipid Peroxidation, which can lead to protein aggregates such as α-synuclein as well as misfolded amyloid β peptide, the latter becoming an amyloid β (Aβ) plaque affecting neuron signaling induced by (4) Cholinergic Insufficiency. In turn, accumulation of Aβ plaque induces (5) Microglia Activation with the concomitant release of (6) Inflammatory Cytokines and produces neuroinflammation. On the other hand, (7) Dysregulation of Ca2+ because of neuronal membrane depolarization could induce synaptic deficits and promote the accumulation of Aβ plaques, and (8) Neurofibrillary Tangles through calpain activation. In addition, sustained calcium inflow results in over-activation of neuronal nitric oxide synthase (nNOS), with the increase in nitric oxide synthesis leading to oxidative stress/nitrosative stress and generalized brain inflammation. Moreover, ROS accumulation induces (9) kinases activation (glycogen synthase kinase-3β, GSK-3β) and induces tau hyperphosphorylation, promoting the accumulation of Aβ plaques. Accumulation of Aβ oligomers causes removal of insulin receptors (IRS) from the cell surface, inducing a (10) Neuronal Insulin Resistance and inhibiting the activation of glucose transporter type 4 (GLUT 4). Dysfunctional insulin signaling brings mammalian target of rapamycin (mTOR) pathway down and results in (11) Autophagy failure to accumulate Aβ plaques. Finally, the synthesized cholesterol binds apolipoprotein E (APOE) to form APOE–cholesterol (APOE–CH) particles. APOE–CH particles are internalized into neurons, and the free cholesterol is metabolized to 24-hydroxycholesterol (24-OHC), which subsequently passes through the blood–brain barrier (BBB) and enters into plasma, while plasma (12) 27 hydroxylcholesterol (27-OHC) flows into the brain, increasing the level of α-synuclein and eventually forms Lewy bodies (LBs). Back lines indicate stimulation, while red lines indicate inhibition.