Abstract
This study assessed the association between sarcopenia and metabolic syndrome in Korean adults aged over 50 years. The study obtained data from the Korea National Health and Nutrition Examination Survey (KNHANES, 2008–2011), a cross-sectional and nationally representative survey conducted by the Korean Centers for Disease Control and Prevention. Among the 8363 participants included in this study, the prevalence rate of sarcopenia according to metabolic syndrome was stratified by sex. Crude odds ratios not adjusted for any variables were 1.827 (1.496–2.231) in males, 2.189 (1.818–2.635) in females, and 2.209 (1.766–2.331) in total participants compared with non-sarcopenia. Model 3, which was adjusted for all variables that could affect sarcopenia and metabolic syndrome, showed significant increases in the odds ratios, to 1.957 (1.587–2.413) in males, 1.779 (1.478–2.141) in females, and 1.822 (1.586–2.095) for total participants. The results suggest that the association between sarcopenia and metabolic syndrome is significant in Korean adults.
Keywords: sarcopenia, metabolic syndrome, Korean
1. Introduction
Sarcopenia is described as a loss of muscle mass and strength because of changes in body composition [1]. The mechanism of sarcopenia remains unknown; however, it is related to various conditions such as a lack of activity, malnutrition, aging, and changes in levels of hormones, including cortisol and testosterone [2]. Furthermore, the increased body fat associated with sarcopenia patients’ decreased muscle mass is linked to cardiovascular disease, diabetes, physical impairment, and mortality [3,4].
In Korea, the prevalence of sarcopenia among the elderly, aged 70 or older, was 18.4% in 2017 [5]. In addition, sarcopenia affected more than 50 million adults globally in 2000, and this number is expected to increase to more than 200 million by 2040 [6]. The socioeconomic cost of sarcopenia in the United States was estimated to be $18.5 billion in 2000, and, thus, additional studies on sarcopenia are required [7].
Metabolic syndrome (MetS) is a collection of metabolic disorders (abdominal obesity, hypertension, high blood glucose level, and abnormal blood lipids) related to an elevated risk of cardiovascular morbidity and death, as well as all-cause mortality [8]. Insulin resistance is the most common cause of MetS [9]. As blood glucose levels increase in response to increased insulin resistance, insulin secretion increases further, resulting in hyperinsulinemia, which limits sodium excretion in the kidneys and causes hypertension [10]. It also decreases high-density lipoprotein cholesterol (HDL-C) levels and increases triglyceride (TG) levels, resulting in dyslipidemia [11].
Sarcopenia is a geriatric and aging illness that has been linked to various metabolic diseases, such as obesity, insulin resistance, diabetes, dyslipidemia, and hypertension [12,13,14]. Furthermore, the increased body fat associated with metabolic disorders has been found in people with sarcopenia, owing to reduced muscle mass [3]. An association between sarcopenia and MetS has been suggested by several studies; however, this association remains unclear. Various studies have identified a link between sarcopenia and MetS, while other studies have shown that the relationship is dependent on gender [15,16,17]. However, these studies were only conducted for subjects who were too old or suffered from certain other diseases. Moreover, information on the link between sarcopenia and MetS from population-based studies in Korea is insufficient. In addition, the change in skeletal muscle mass, strength, and power of people aged 30 to 50 is not significant, while the change is noticeable from the age of 50 or older [18,19]. Consequently, we investigated the association between sarcopenia and MetS, by focusing on Korean adults aged ≥50 years, owing to the significant loss of muscle mass in this age group.
2. Materials and Methods
2.1. Data Source and Sampling
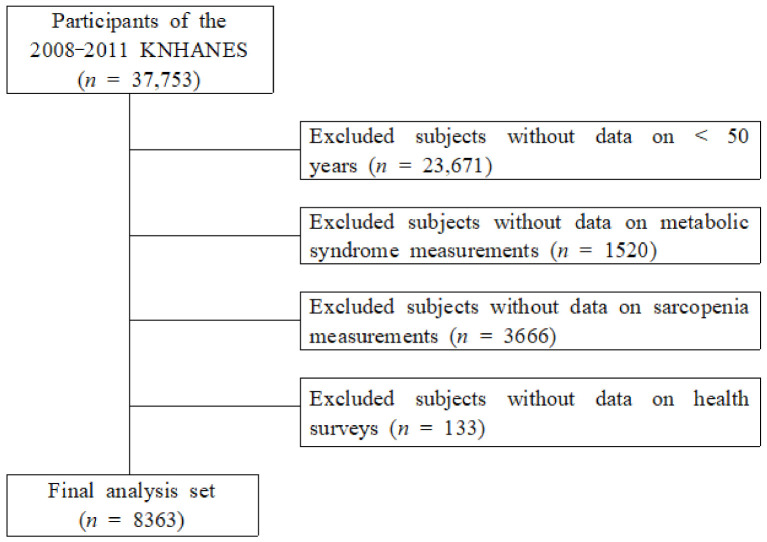
This study used data from the Korea National Health and Nutrition Survey (KNHANES, 2008–2011) conducted by the Korean Centers for Disease Control and Prevention. The participants responded to both an examination and health survey of adults aged 50 years or older. Among 37,753 participants who participated in the KNHANES, there were 23,671 participants aged <50 years, 1520 participants for whom MetS components were not assessed, and 3666 participants for whom sarcopenia variables were not assessed, while 539 non-participants in the health survey were excluded. Consequently, 8363 participants were included in this study (Figure 1).
Figure 1.
Selection of participants from the Korea National Health and Nutrition Examination Survey 2008–2011.
2.2. Measurement of Variables
2.2.1. Covariates
BMI was calculated by dividing weight (kg)/height (m2). Smoking status was categorized as never smokers, ex-smokers, and current smokers. Drinking condition was dichotomized into current users and non-users. Marital status was classified as living with a spouse or without a spouse. Individual income was divided into quartiles. Physical examinations included height, weight, fasting glucose, waist circumference, triglyceride, diastolic and systolic blood pressure, HDL-cholesterol (HDL-C), and total cholesterol measurement variables. Blood samples were collected from participants in the morning after overnight fasting and were analyzed at a national central laboratory. Blood pressure was measured using a mercury sphygmomanometer, with participants in a seated position after a 10 min rest period. Two measurements were made for all participants at 5 min intervals. An average of the two measurements was used for data analyses.
2.2.2. Sarcopenia
Appendicular skeletal muscle mass (ASM) was measured using dual X-ray absorptiometry (QDR4500A; Hologic, Inc., Bedford, MA, USA). The sarcopenia muscle mass index (SMI) was calculated as ASM (kg)/body mass index (BMI, kg/m2). Sarcopenia was defined as an SMI <0.789 in males and <0.521 in females, based on the criteria of the Sarcopenia Project [6].
2.2.3. Metabolic Syndrome
MetS in the study was diagnosed if three or more of the five components were satisfied using the guidelines of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III): (1) abdominal obesity: waist circumference >90 cm in men and >85 cm in women; (2) hypertriglyceridemia: ≥150 mg/dL; (3) reduced HDL-C: <40 mg/dL for men and <50 mg/dL for women; (4) hypertension: systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg; and (5) elevated fasting glucose: ≥100 mg/dL. If participants were using anti-hypertension, diabetes, or dyslipidemia treatment medication, they were present [20].
2.3. Data Analysis
Since this study uses complex sample data, the weighting given by the KNHANES has been applied. Data were expressed as absolute numbers and estimated percentages (with standard errors) or as mean ± standard deviation (SD). The survey responses were weighted by reference to a multistage, complex probability sampling design. General characteristics were compared according to sarcopenia and MetS using the chi-square test. Multivariate logistic regression analysis was used to analyze the association between sarcopenia and spirometry patterns and p-values <0.05 were considered statistically significant. Data analysis was performed using SPSS 27.0 Window’s version.
3. Results
3.1. Characteristics of Participants According to Sarcopenia and Sex
Both males and females showed a significantly higher prevalence of MetS in participants with sarcopenia (Sarcopenia/Non-sarcopenia: 49.6/35.0 in males, 55.1/35.9 in females). In men, all variables showed significance, except for individual income, diastolic blood pressure, and total cholesterol. In contrast, significance was shown in all variables, except smoking status, individual income, weight, and diastolic blood pressure, in females (Table 1).
Table 1.
Participant characteristics according to sarcopenia and sex.
| Variables | Males | Females | ||||
|---|---|---|---|---|---|---|
| Sarcopenia (n = 681) |
Non-Sarcopenia (n = 2946) |
p | Sarcopenia (n = 951) |
Non-Sarcopenia (n = 3785) |
p | |
| Age (y) | 64.75 ± 0.41 | 59.93 ± 0.19 | <0.0001 | 66.10 ± 0.42 | 61.04 ± 0.19 | <0.0001 |
| MetS, n (%) | 331 (49.6) | 978 (35.0) | <0.0001 | 527 (55.1) | 1372 (35.9) | <0.0001 |
| BMI (kg/m2), mean (SD) | 25.01 ± 0.13 | 23.61 ± 0.07 | <0.0001 | 26.07 ± 0.16 | 23.81 ± 0.06 | <0.0001 |
| <18.5 (underweight), n (%) | 8 (1.2) | 120 (3.4) | 3 (0.7) | 118 (2.9) | ||
| <25 (normal-weight), n (%) | 346 (48.3) | 1982 (65.4) | 375 (40.8) | 2454 (64.7) | ||
| ≥25 (overweight), n (%) | 327 (50.5) | 844 (1.0) | 573 (58.5) | 1213 (32.4) | ||
| Smoking status, (%) (current-/ex-/non-smoker) |
56.4/31.1/12.4 | 60.3/23.4/16.2 | 0.002 | 6.5/2.7/90.9 | 7.2/1.6/91.2 | 0.200 |
| Drinking status (%) (current-/non-drinking) |
64.5/35.5 | 71.6/28.4 | 0.002 | 22.9/77.1 | 28.4/71.6 | 0.005 |
| Marital status, (%) (living with spouse) |
88.3 | 92.7 | 0.003 | 55.0 | 70.3 | <0.0001 |
| Income (individual) | ||||||
| Q1 (lowest) Q2 Q3 Q4 (highest) |
25.7 27.2 26.3 20.8 |
24.0 25.5 24.0 26.5 |
0.121 | 26.5 23.6 27.9 21.9 |
24.4 25.2 25.5 24.9 |
0.221 |
| Height (cm) | 160.85 ± 0.25 | 168.35 ± 0.12 | <0.0001 | 148.14 ± 0.22 | 155.07 ± 0.12 | <0.0001 |
| Weight (kg) | 64.91 ± 0.43 | 67.05 ± 0.24 | <0.0001 | 57.36 ± 0.41 | 57.36 ± 0.17 | 0.996 |
| Fasting glucose (mg/dL) | 109.79 ± 1.48 | 104.53 ± 0.55 | 0.001 | 103.81 ± 1.03 | 100.49 ± 0.45 | 0.003 |
| Waist circumference (cm) | 87.98 ± 0.37 | 84.95 ± 0.20 | <0.0001 | 86.42 ± 0.42 | 81.43 ± 0.21 | <0.0001 |
| Triglyceride | 183.29 ± 6.95 | 160.78 ± 3.33 | 0.005 | 148.50 ± 3.14 | 133.89 ± 1.78 | <0.0001 |
| Systolic BP (mmHg) | 130.44 ± 0.87 | 126.27 ± 0.44 | <0.0001 | 131.30 ± 0.74 | 126.06 ± 0.42 | <0.0001 |
| Diastolic BP (mmHg) | 79.90 ± 0.57 | 80.78 ± 0.29 | 0.143 | 77.94 ± 0.39 | 77.83 ± 0.23 | 0.802 |
| HDL-C | 43.82 ± 0.53 | 45.42 ± 0.28 | 0.005 | 47.94 ± 0.46 | 48.70 ± 0.26 | <0.0001 |
| Total cholesterol | 185.81 ± 1.97 | 187.59 ± 0.79 | 0.400 | 205.42 ± 1.36 | 199.76 ± 0.78 | <0.0001 |
| Skeletal muscle index | 0.74 ± 0.00 | 0.91 ± 0.00 | <0.0001 | 0.48 ± 0.00 | 0.61 ± 0.00 | <0.0001 |
Data are presented as means ± SD or number (%). MetS; metabolic syndrome, BMI; body mass index, BP; blood pressure HDL-C; high density lipoprotein-cholesterol; p-value using ANOVA or chi-square test.
3.2. Characteristics of Participants According to MetS and Sex
In this study, the prevalence of sarcopenia according to MetS differed in both males and females (MetS/Non-MetS: 22.0/13.4 in males, 27.3/14.7 in females). Age, smoking and drinking status, marital status, individual income, and total cholesterol did not show significance in males. However, only smoking status did not show significance in females (Table 2).
Table 2.
Participant characteristics according to metabolic syndrome and sex.
| Variables | Males | Females | ||||
|---|---|---|---|---|---|---|
| MetS (n = 1309) |
Non-MetS (n = 2318) |
p | MetS (n = 1899) |
Non-MetS (n = 2837) |
p | |
| Age (y) | 60.38 ± 0.26 | 60.94 ± 0.22 | 0.097 | 64.47 ± 0.28 | 60.43 ± 0.21 | <0.0001 |
| Sarcopenia, n (%) | 331 (22.0) | 350 (13.4) | <0.0001 | 527 (27.3) | 424 (14.7) | <0.0001 |
| BMI (kg/m2), mean (SD) | 25.37 ± 0.10 | 22.93 ± 0.08 | <0.0001 | 25.80 ± 0.10 | 23.23 ± 0.06 | <0.0001 |
| <18.5 (underweight), n (%) | 9 (0.6) | 119 (4.5) | 12 (0.7) | 109 (3.6) | ||
| <25 (normal-weight), n (%) | 581 (43.1) | 1747 (74.1) | 759 (40.9) | 2070 (72.6) | ||
| ≥25 (overweight), n (%) | 719 (56.3) | 452 (21.4) | 1128 (58.4) | 658 (23.8) | ||
| Smoking status, (%) (current-/ex-/non-smoker) |
61.2/23.7/15.1 | 58.8/25.4/15.9 | 0.463 | 7.7/1.8/90.5 | 6.6/1.8/91.6 | 0.528 |
| Drinking status (%) (current-/non-drinking) |
71.6/28.4 | 69.7/30.3 | 0.344 | 23.0/77.0 | 30.2/69.8 | <0.0001 |
| Marital status, (%) (living with spouse) |
90.9 | 92.7 | 0.085 | 60.4 | 71.9 | <0.0001 |
| Income (individual) | ||||||
| Q1 (lowest) Q2 Q3 Q4 (highest) |
24.6 25.0 23.8 26.6 |
24.1 26.3 24.7 24.9 |
0.733 | 26.6 26.6 24.3 22.6 |
23.6 23.7 27.2 25.5 |
0.014 |
| Height (cm) | 167.63 ± 0.212 | 166.79 ± 0.16 | 0.002 | 153.30 ± 0.18 | 153,98 ± 0.15 | 0.003 |
| Weight (kg) | 71.39 ± 0.35 | 63.89 ± 0.25 | <0.0001 | 60.73 ± 0.26 | 55.14 ± 0.18 | <0.0001 |
| Fasting glucose (mg/dL) | 116.13 ± 0.21 | 98.99 ± 0.55 | <0.0001 | 111.38 ± 0.82 | 94.41 ± 0.37 | <0.0001 |
| Waist circumference (cm) | 90.30 ± 0.28 | 82.55 ± 0.21 | <0.0001 | 87.76 ± 0.26 | 78.89 ± 0.21 | <0.0001 |
| Triglyceride | 230.53 ± 5.13 | 125.05 ± 3.16 | <0.0001 | 184.88 ± 2.84 | 105.06 ± 1.29 | <0.0001 |
| Systolic BP (mmHg) | 133.58 ± 0.55 | 123.01 ± 0.47 | <0.0001 | 135.61 ± 0.46 | 121.48 ± 0.45 | <0.0001 |
| Diastolic BP (mmHg) | 84.31 ± 0.38 | 78.43 ± 0.30 | <0.0001 | 80.91 ± 0.29 | 75.83 ± 0.26 | <0.0001 |
| HDL-cholesterol | 39.79 ± 0.33 | 48.36 ± 0.32 | <0.0001 | 42.74 ± 0.25 | 52.37 ± 0.29 | <0.0001 |
| Total cholesterol | 188.97 ± 1.34 | 186.30 ± 0.85 | 0.088 | 202.74 ± 1.17 | 199.65 ± 0.84 | 0.030 |
| Skeletal muscle index | 0.86 ± 0.00 | 0.90 ± 0.00 | <0.0001 | 0.56 ± 0.00 | 0.60 ± 0.00 | <0.0001 |
Data are presented as means ± SD or number (%). MetS; metabolic syndrome, BMI; body mass index, BP; blood pressure HDL-C; high density lipoprotein-cholesterol; p-value using ANOVA or chi-square test.
3.3. MetS Components According to Sarcopenia and Sex
The five components of MetS, namely high fasting glucose level, abdominal obesity, high TG level, high blood pressure, and low HDL-C level, were all higher in males with sarcopenia. However, there were no significant differences in HDL-C levels in female (Table 3).
Table 3.
Metabolic syndrome components according to sarcopenia and sex.
| Variables | Males | p | Females | p | ||
|---|---|---|---|---|---|---|
| Sarcopenia | Non-Sarcopenia | Sarcopenia | Non-Sarcopenia | |||
| a High fasting glucose | 55.3 ± 2.2 | 44.9 ± 1.1 | <0.0001 | 46.2 ± 2.1 | 34.9 ± 0.9 | <0.0001 |
| b Abdominal obesity | 41.5 ± 2.4 | 27.5 ± 1.0 | <0.0001 | 54.3 ± 2.2 | 34.4 ± 1.1 | <0.0001 |
| c High triglyceride | 47.3 ± 2.1 | 38.4 ± 1.1 | <0.0001 | 40.3 ± 2.0 | 30.0 ± 1.0 | <0.0001 |
| d High blood pressure | 56.7 ± 2.5 | 48.4 ± 1.2 | 0.002 | 55.9 ± 2.1 | 44.9 ± 1.1 | <0.0001 |
| e Low HDL-C | 45.0 ± 2.5 | 36.9 ± 1.2 | 0.002 | 62.0 ± 1.9 | 59.3 ± 1.1 | 0.213 |
| MetS | 49.6 ± 2.3 | 35.0 ± 1.1 | <0.0001 | 55.1 ± 2.0 | 35.9 ± 1.0 | <0.0001 |
Data were presented as means ± SD or number (%). HDL-C; high density lipoprotein-cholesterol, MetS; metabolic syndrome; a High fasting glucose level is defined as FBG ≥100 mg/dL; b Abdominal obesity is defined as waist circumference >90 cm (male) or >85 cm (female); c High triglyceride level is defined as TG ≥150 mg/dL; d Low HDL-C level is defined as HDL-C <40 mg/dL (male) or <50 mg/dL (female); e High blood pressure is defined as SBP ≥130 mmHg or DBP ≥85 mmHg.
3.4. Odds Ratios for Sarcopenia According to MetS Stratified by Sex
The prevalence of sarcopenia according to MetS was classified according to sex. Crude odds ratios that were not adjusted for any variables showed that the probability of sarcopenia was 1.827 (1.496–2.231) in males, 2.189 (1.818–2.635) in females, and 2.209 (1.766–2.331) for the total participants, compared with non-sarcopenia. In model 3, which was adjusted for variables that could affect sarcopenia and MetS, significant increases in the probabilities were observed: 1.957 (1.587–2.413) in males, 1.779 (1.478–2.141) in females, and 1.822 (1.586–2.095) for total participants (Table 4).
Table 4.
Odds ratios for sarcopenia according to metabolic syndrome stratified by sex.
| MetS | OR (95% CI) | p | |
|---|---|---|---|
| Crude | Males | 1.827 (1.496–2.231) | <0.0001 |
| Females | 2.189 (1.818–2.635) | <0.0001 | |
| Total | 2.029 (1.766–2.331) | <0.0001 | |
| Model 1 | Males | 1.970 (1.597–2.430) | <0.0001 |
| Females | 1.792 (1.489–2.157) | <0.0001 | |
| Total | 1.849 (1.608–2.126) | <0.0001 | |
| Model 2 | Males | 1.972 (1.599–2.432) | <0.0001 |
| Females | 1.794 (1.489–2.160) | <0.0001 | |
| Total | 1.822 (1.586–2.095) | <0.0001 | |
| Model 3 | Males | 1.957 (1.587–2.413) | <0.0001 |
| Females | 1.779 (1.478–2.141) | <0.0001 | |
| Total | 1.822 (1.586–2.095) | <0.0001 |
Model 1: Adjusted for age; Model 2: Model 1 + smoking and drinking status; Model 3: Model 2 + individual income and marital status; Reference category: non-sarcopenia.
4. Discussion
In this study of Korean adults aged over 50 years, the main findings are that sarcopenia is independently related to MetS, after adjusting for various variables including age, sex, smoking and drinking status, and individual income.
Both males and females had a significantly higher average age in people with sarcopenia. This result can be interpreted to mean that muscle mass decreases with age. This is consistent with the findings of a previous study, namely a gradual decrease in muscle mass was noted after reaching the maximum muscle mass in people in their 30’s, a 1–2% loss of muscle mass is noted every year in those in their 50’s, and the muscle mass is reduced by half in those in their 70’s [21].
In Table 2, the characteristics of participants according to the prevalence of MetS showed significant differences between males and females in the prevalence of sarcopenia. Table 3 shows significant differences in all variables in males, and significant differences in all variables except HDL-C in females in the analysis of MetS components following sarcopenia. The relationship analysis of sarcopenia with MetS in Table 4 shows that the association is also high at 1.822 (1.586–2.095) in model 3, which adjusted the variables that could affect it. Therefore, a correlation between the two was evident.
A previous study revealed that the association between sarcopenia and MetS was only found in men, which was different from the results of this study [17]. This difference is thought to be due to the age of the study subjects. In this previous study, the average age of the study subjects was 73.1 years in males and 72.8 years in females, and the proportion of the elderly in their 70’s or older was high. In addition, in the previous study, the age group of the subjects was high, regardless of sarcopenia, so there was no significant difference between groups in the MetS factor excluding WC [17]. For this reason, it is thought that there may have been a difference from the results of this study. Another study also revealed that there was only association between sarcopenia and metabolic syndrome in males, but it is believed that there was a difference from our study, as the subjects of this study were limited to chronic obstructive pulmonary disease patients [15].
Although the pathological mechanisms underlying the relationship between sarcopenia and MetS remain unknown, there may be several shared causes. First, the five components of MetS are abdominal obesity, high blood pressure, high blood glucose, high TG, and low HDL-C [22]. Insulin resistance and inflammation have been hypothesized to be the primary factors underlying MetS [20]. Under normal conditions, skeletal muscle is essential for systemic glucose homeostasis, accounting for 80% of normal glucose absorption and metabolism via insulin stimulation [23]. Second, IL-6, IL-8, IL-15, fibroblast growth factor 21, irisin, myonectin, and myostatin are among the myokines produced and secreted by skeletal muscle cells [24]. Exercise and muscle exertion control the majority of myokines. They have positive effects on glucose and lipid metabolism, as well as inflammation, by counteracting the negative effects of inflammatory cytokines [25]. Third, by lowering the basal metabolic rate, a reduction in muscle mass leads to an increase in fat mass [26]. Increased fat levels cause inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin (IL)-634, to be secreted, as well as insulin resistance, which contribute to MetS [27,28]. Thus, a decrease in muscle mass results in an increase in insulin resistance, which leads to MetS and type 2 diabetes, as it is the major location for glucose and fatty acid metabolism. The metabolic effect of adipokines secreted by adipose tissue has been shown to reverse the pathological consequences of metabolic disorders by secreting proteins or myokines from skeletal muscle fibers (especially type IIb muscle fibers), and these muscle fibers are selectively lost during the aging process [29].
Despite several significant findings, this study had some limitations. First, it was a cross-sectional study that assessed both sarcopenia and MetS variables simultaneously; thus, while this study may provide additional insights into the nature of this association, it was difficult to determine the order of the underlying causes of sarcopenia, because a cross-sectional study design tends to cause confusion regarding the temporal sequence. Therefore, identifying a method to elucidate the causal relationship between sarcopenia and MetS through longitudinal research will be important in the future. Second, because there were no KNHANES data on types and dosage of medication, medicines that may impact sarcopenia and MetS could not be ruled out. Third, there was a draw-recall bias because the socio-statistical characteristics of the research population were gathered through questionnaires. Finally, because the information on the components of MetS was inadequate, the total prevalence of MetS may have been underestimated. However, this process was most likely randomly eliminated and is unlikely to have had a major influence on the study results. Despite these limitations, the scholarly and clinical implications of this study are substantial. The strength of this study is that it collected data from a large Korean population with a high response rate, allowing for numerous statistical modifications to account for potential causes of disruption.
5. Conclusions
To summarize the study results, after adjusting for different confounding variables, such as age, sex, smoking and drinking behavior, and individual income, the primary finding of this study of Korean adults aged over 50 years was that sarcopenia is independently related to MetS.
Author Contributions
Conceptualization, S.S.; methodology, D.-Y.L.; software, D.-Y.L.; validation, S.S. and D.-Y.L.; formal analysis, D.-Y.L.; investigation, D.-Y.L.; resources, S.S.; data curation, S.S. and D.-Y.L.; writing—original draft preparation, D.-Y.L.; writing—review and editing, S.S. and D.-Y.L.; visualization, S.S. and D.-Y.L.; supervision, S.S.; project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1A6A1A03040177).
Institutional Review Board Statement
The Korea National Health and Nutrition Examination Survey is survey that does not require an ethics review and corresponds to the research conducted by the government for public welfare, in accordance with Article 2, Paragraph 1 of the Bioethics Act, and Article 2, Paragraph 1 of the Enforcement Rule of the same act. Conducted without deliberation of the committee. Hence, ethical review and approval for this study were waived.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
All data were anonymized and can be downloaded from the website at https://knhanes.kdca.go.kr/knhanes (accessed on 12 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127:990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Bai L. Sarcopenia in the elderly: Basic and clinical issues. Geriatr. Gerontol. Int. 2012;12:388–396. doi: 10.1111/j.1447-0594.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim K.M., Lim S., Choi S.H., Kim J.H., Shin C.S., Park K.S., Jang H.C. Cardiometabolic implication of sarcopenia: The Korea National Health and Nutrition Examination Study (KNHANES) 2008–2010. IJC Metab. Endocr. 2014;4:63–69. doi: 10.1016/j.ijcme.2014.06.001. [DOI] [Google Scholar]
- 4.Janssen I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.Bae E.J., Kim Y.H. Factors Affecting Sarcopenia in Korean Adults by Age Groups. Osong. Public. Health Res. Perspect. 2017;8:169–178. doi: 10.24171/j.phrp.2017.8.3.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen I., Shepard D.S., Katzmarzyk P.T., Roubenoff R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 8.Isomaa B., Almgren P., Tuomi T., Forsén B., Lahti K., Nissén M., Taskinen M.R., Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 9.Gami A.S., Witt B.J., Howard D.E., Erwin P.J., Gami L.A., Somers V.K., Montori V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Reaven G.M., Laws A. Insulin resistance, compensatory hyperinsulinaemia, and coronary heart disease. Diabetologia. 1994;37:948–952. doi: 10.1007/BF00400953. [DOI] [PubMed] [Google Scholar]
- 11.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doğan M.H., Karadag B., Ozyigit T., Kayaoglu S., Ozturk A.O., Altuntas Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clin. Belg. 2012;67:328–332. doi: 10.2143/acb.67.5.2062685. [DOI] [PubMed] [Google Scholar]
- 13.Khamseh M.E., Malek M., Aghili R., Emami Z. Sarcopenia and diabetes: Pathogenesis and consequences. Br. J. Diabetes Vasc. Dis. 2011;11:230–234. doi: 10.1177/1474651411413644. [DOI] [Google Scholar]
- 14.Waters D.L., Baumgartner R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011;27:401–421. doi: 10.1016/j.cger.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Chung J.H., Hwang H.J., Han C.H., Son B.S., Kim D.H., Park M.S. Association between sarcopenia and metabolic syndrome in chronic obstructive pulmonary disease: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011. COPD. 2015;12:82–89. doi: 10.3109/15412555.2014.908835. [DOI] [PubMed] [Google Scholar]
- 16.Moon S.-S. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr. J. 2013;61:EJ13-0244. doi: 10.1507/endocrj.EJ13-0244. [DOI] [PubMed] [Google Scholar]
- 17.Ishii S., Tanaka T., Akishita M., Ouchi Y., Tuji T., Iijima K., Investigators K.S. Metabolic syndrome, sarcopenia and role of sex and age: Cross-sectional analysis of Kashiwa cohort study. PLoS ONE. 2014;9:e112718. doi: 10.1371/journal.pone.0112718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proctor D.N., Balagopal P., Nair K.S. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J. Nutr. 1998;128:351s–355s. doi: 10.1093/jn/128.2.351S. [DOI] [PubMed] [Google Scholar]
- 19.Keller K., Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013;3:346–350. doi: 10.32098/mltj.04.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen K.F., Dufour S., Savage D.B., Bilz S., Solomon G., Yonemitsu S., Cline G.W., Befroy D., Zemany L., Kahn B.B., et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balagopal P., Rooyackers O.E., Adey D.B., Ades P.A., Nair K.S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997;273:E790-800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 22.Romeo G.R., Lee J., Shoelson S.E. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen K.F., Shulman G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002;90:11g–18g. doi: 10.1016/S0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Ballantyne C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017;127:43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckardt K., Görgens S.W., Raschke S., Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57:1087–1099. doi: 10.1007/s00125-014-3224-x. [DOI] [PubMed] [Google Scholar]
- 26.Roubenoff R. Sarcopenic obesity: Does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann. N. Y. Acad. Sci. 2000;904:553–557. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 27.Reaven G.M. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 28.Schrager M.A., Metter E.J., Simonsick E., Ble A., Bandinelli S., Lauretani F., Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh K. Adipokines, myokines and cardiovascular disease. Circ. J. 2009;73:13–18. doi: 10.1253/circj.CJ-08-0961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were anonymized and can be downloaded from the website at https://knhanes.kdca.go.kr/knhanes (accessed on 12 December 2021).