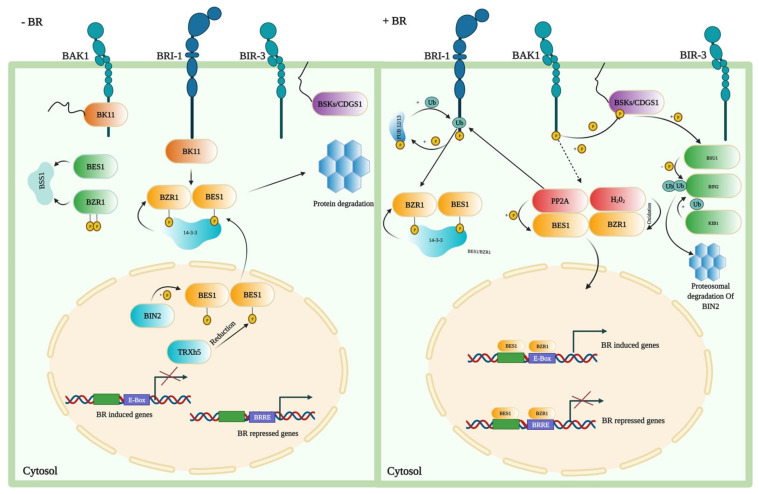
Figure 3.
Signaling in the absence and presence of BRs in A. thaliana. When BRs are absent, BZR1 and BES1 proteins are being phosphorylated by the BIN2 that activates them by promoting binding of these proteins to the 14-3-3 proteins, resulting in cytoplasmic retention and degradation. This enhances the cytoplasmic retention of TFs, preventing them from entering the nucleus and terminating the response induced by the BR. When BRs are present, BR binding to BRI1 and the co-receptor BAK1 causes BKI1 to dissociate from BRI1 and causes trans-phosphorylation between BRI1 and BAK1. Through direct phosphorylation, the activated BRI1–BAK1 receptor complex transmits its signal to BSKs and Constitutive differential growth 1 (CDG1). BSU1 phosphatase is activated by BSKs or CDG1. BSU1 subsequently dephosphorylates the BIN2 to inactive it, and the E3 ligase KIB1 mediates the degradation of BIN2. Meanwhile, PP2A dephosphorylates BZR1 and BES1 to activate them, allowing TFs to enter the nucleus and regulate the expression of the BR target genes, either by direct interaction or through interactions with other TFs. Moreover, PP2A positively regulates BR signaling by the dephosphorylation of BZR1 and BES1, while the SBI1 (Suppressor of BRI1) deactivates the BRI1 through PP2A methylation.