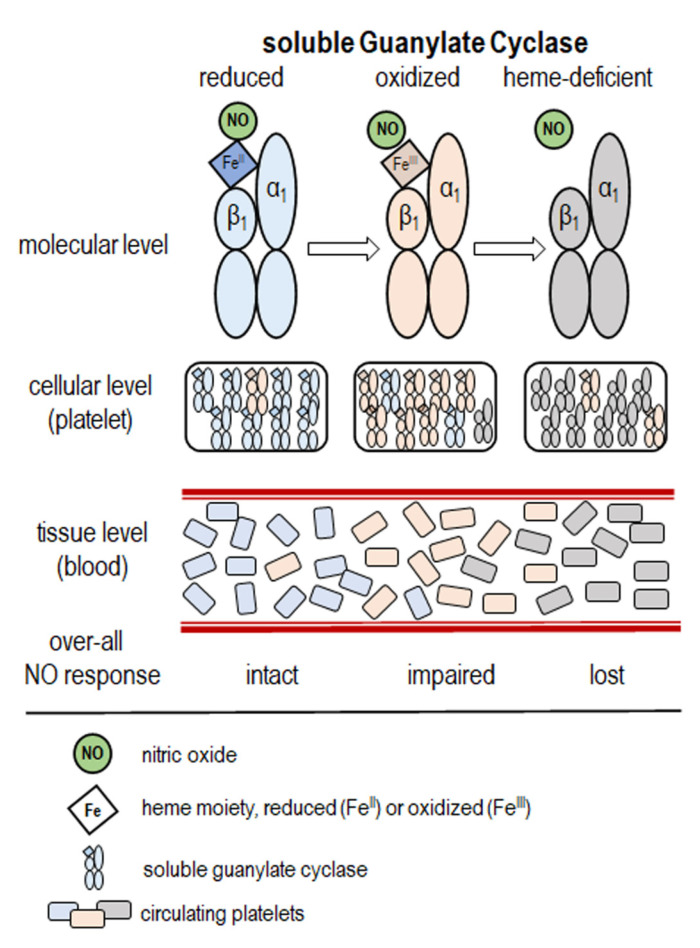
Figure 3.
Bases for variability in platelet soluble guanylate cyclase (sGC) functionality. sGC is a heterodimer that is composed of one α and one heme-binding β subunit. In its FeII form, this heme moiety is the target of nitric oxide (NO), either endogenous (synthesized by endothelium) or exogenous. The binding of NO to the heme results in the activation of the catalytic domain, which produces cGMP from GTP. In conditions of oxidative stress, heme is oxidized to FeIII form, which has a lesser affinity for NO, resulting in diminished activation of sGC (“NO resistance”) and a loss of the heme moiety. Within platelets, there is a heterogeneity of the sGC molecular state (reduced, oxidized, and heme-deficient). The overall platelet responsiveness to NO in vivo reflects the relative prevalence of each of these sGC states.