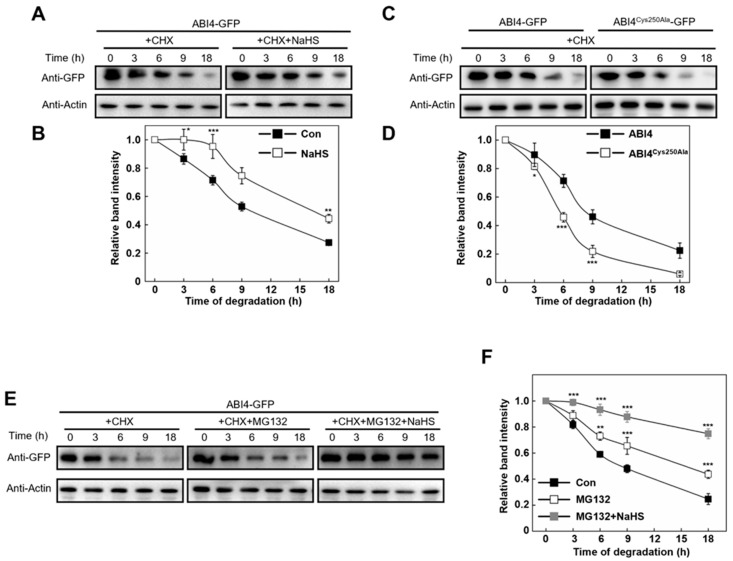
Figure 5.
The stability of ABI4 is regulated by its persulfidation In vivo. (A) The effect of NaHS on the stability of ABI4 In vivo. Arabidopsis protoplasts isolated from the Col-0 lines were transfected with ABI4-GFP expressing plasmid constructs. After incubation in low light for 12 h, protoplasts were treated with 0.1 mM NaHS (or distilled water, as a control) and 150 μM CHX (to block protein translation) for 1 h. Then protoplasts were lysed and incubated at 30 °C after being divided into five tubes. Samples were stopped at indicated time points and checked by immunoblot analysis. Proteins were detected using an anti-GFP antibody, and relative amounts of proteins were determined by densitometry normalized to actin. (B) Quantification of the relative band intensity shown in (A). (C) The stability of wild-type ABI4 or its Cys250Ala mutated version In vivo. Arabidopsis protoplasts isolated from the wild-type lines were transfected with ABI4-GFP or ABI4Cys250Ala-GFP expressing plasmid constructs, respectively. Sample harvest and measurements were as described for (A). (D) Quantification of the relative band intensity shown in (C). (E) The effect of MG132 and NaHS on the stability of ABI4 In vivo. Protoplasts were treated with 50 µm MG132 or 50 µm MG132 and 0.1 mM NaHS, respectively (or distilled water, as a control) for 1 h, and other sample treatments and measurements were as described for (A). (F) Quantification of the relative band intensity shown in (E). For the quantification of relative band intensity, the data indicate the relative abundance of the corresponding protein compared with that of the control sample (set to 1.0). Signals from two independent experiments were quantified. Asterisks represent significant differences between treatments according to Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001).