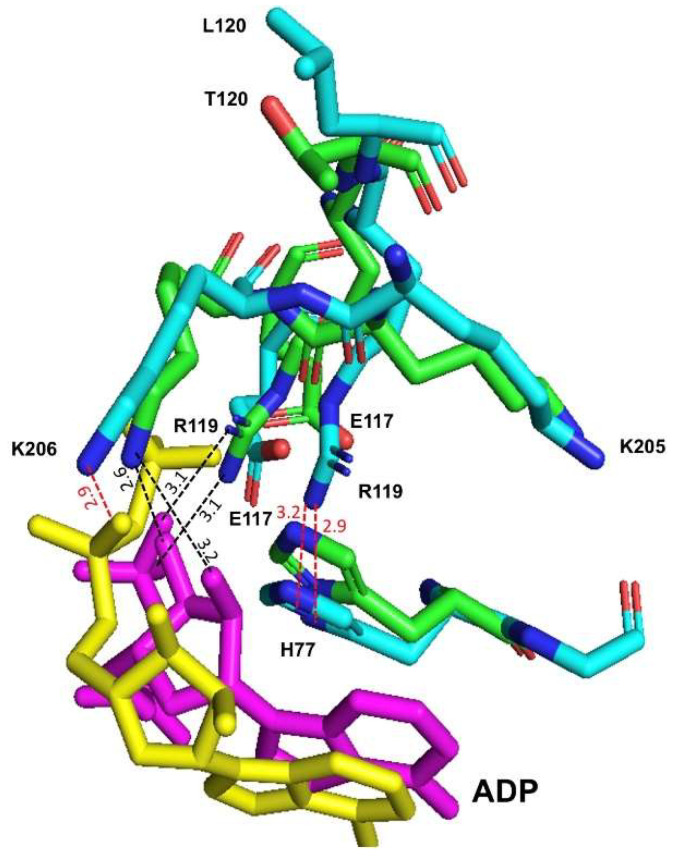
Figure 8.
Comparison of the interactions of ADP (magenta and yellow) in the active site of the WT-RMPK (green) and T120L mutant (cyan). In the WT-RMPK, NH2 of R119 is at H-bond distance (3.13 and 3.11 Å) of O3B and O1B of ADP, respectively. In contrast, in the T120L mutant, NH2 of R119 is at 2.92 and 3.16 Å of ND1 and CE1 of H77, respectively. In WT-RMPK, NZ of K206 is at H-bond distance (2.57 and 3.19 Å) of O3´ and O2´of ribose, respectively; whereas in T120L mutant, NZ of K206 is at H-bond distance (2.96 Å) of O1A of ADP. This figure was prepared using the molecular dynamic models of both enzymes in the initial structures described in Figure 7 from PDB 149A.