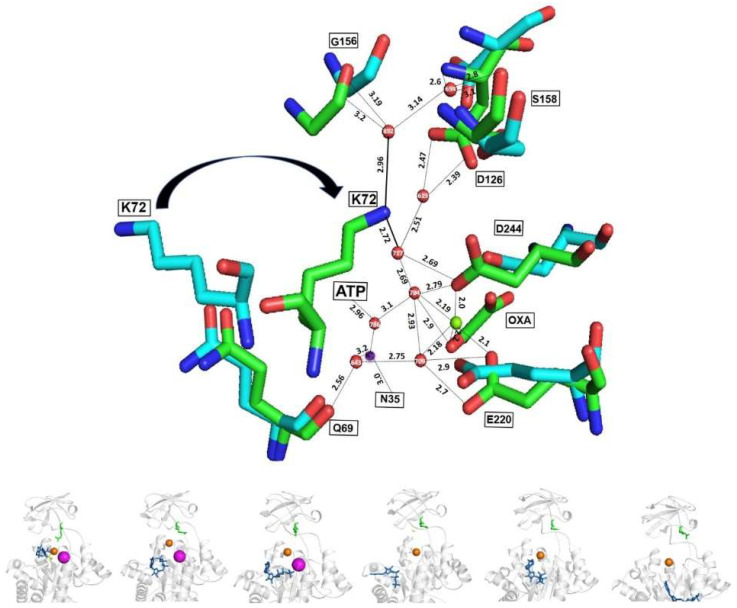
Figure 11.
Ribbon representation of the active sites of MtbPK in the holo (green) and the apoenzyme (cyan); and MD. Superposition of the coordinates of the holo- (PDB 5WS9 subunit A; green) to the apoenzyme (PDB 5WRP subunit A; cyan) are shown. As observed in Figure 9, K72 flips out or into the active site in the apo- and holoenzyme, respectively. ε-amino group of K72 interacts with Mg2+, Oxalate, Mg-ATP, K+, residues of A domain and of the lid (B domain) via a water network. Numbering is according to MtbPK. The bottom part shows the structural models of the MD of MtbPK every 20 ns, observing the same displacement of the K72 (green sticks). Ligands are represented as follows: ATP (blue sticks) Oxalate (yellow sticks), Mg2+ (orange spheres) and K+ (magenta spheres).