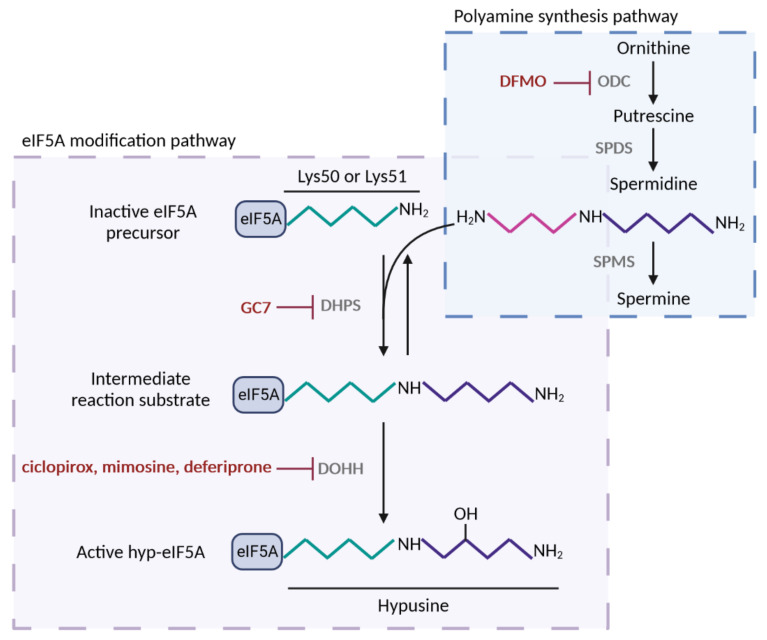
Figure 1.
Polyamine-hypusine pathway and its pharmacological inhibitors. Spermidine substrate for eIF5A hypusination is obtained by the conversion of the polyamine ornithine in putrescine by the enzyme ornithine decarboxylase (ODC); next, spermidine is synthesized from putrescine by spermidine synthase (SPDS). Alternatively, spermidine is converted in spermine by spermine synthase (SPMS). Hypusine modification of lysine-50 (human) or lysine-51 (yeast) residue of eIF5A occurs by the addition of spermidine via two consecutive enzymatic reactions. First, deoxyhypusine synthase (DHPS) transfers the aminobutyl group of spermidine to the amino group of lysine generating an intermediate substrate, which does not accumulate. Second, deoxyhypusine hydroxylase (DOHH) adds a hydroxyl group and forms the hypusine residue of eIF5A, which confers the activity to the protein. eIF5A post-translational modification can be suppressed by inhibitors of DHPS and DOHH, but also by inhibition of ODC, the rate limiting enzyme for spermidine biosynthesis. Figure processing was carried out using BioRender software.