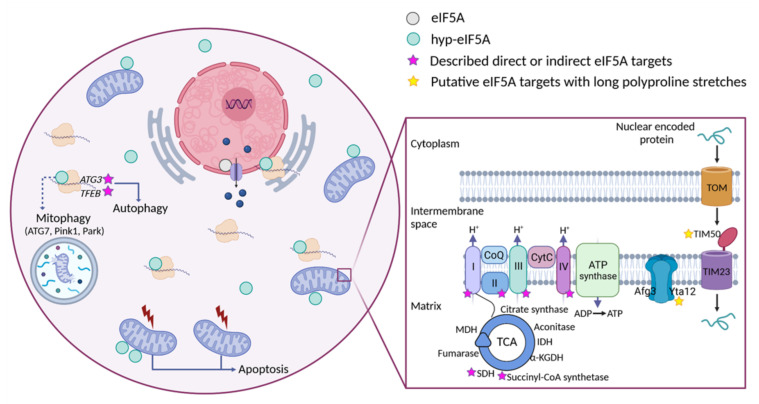
Figure 3.
Cellular funtions of eIF5A and model for its role in maintaining mitochondrial activity. eIF5A is known to be implicated in different cellular processes, although the most relevant and mitochondrial-related of these are represented in the Figure. Bound to ribosomes, hyp-eIF5A facilitates translation elongation at specific motifs [10,11], as well as ER-coupled translation [23,25,26]. In the nucleus, eIF5A helps to export certain mRNAs and proteins [141]. eIF5A plays a controversial role in apoptosis, as it has been defined to be necessary to induce the mitochondrial mediated apoptosis [124,146], but also to lead to cell death when inhibited [133]. Hyp-eIF5A promotes autophagy through the translation of the autophagy factors ATG3 (autophagy-related 3) and TFEB (transcription factor EB) [42,43]. Increasing evidence shows a direct link between hyp-eIF5A and mitochondrial function. In addition to its association with mitochondria [143,144,145,146], some proteins of both the TCA and ETC have been described to be directly or indirectly affected under hyp-eIF5A inhibition [126,147]. It has also been proposed that hyp-eIF5A could mediate mitophagy through ATG7 (Autophagy Related 7), Pink1 (the mitophagy-associated PTEN-induced putative kinase), and Park (the E3 ubiquitin ligase Parkin) proteins [170]. Other proteins involved in the mitochondrial transport of nuclear-encoded proteins and mitochondrial organization are considered as putative eIF5A targets (Figure 2). Among these, the mitochondrial inner-membrane integral proteins Yta12 (protease of the Yta12/Afg3 complex and yeast homolog of human AFG3L2), and Tim50 (essential subunit of the TIM23 complex and yeast homolog of human TIMM50) contain long polyproline stretches in their amino acids sequences, suggesting a possible dependence on eIF5A for their translation and, thus, a possible link between hyp-eIF5A and mitochondrial function. Figure processing was carried out using BioRender software.