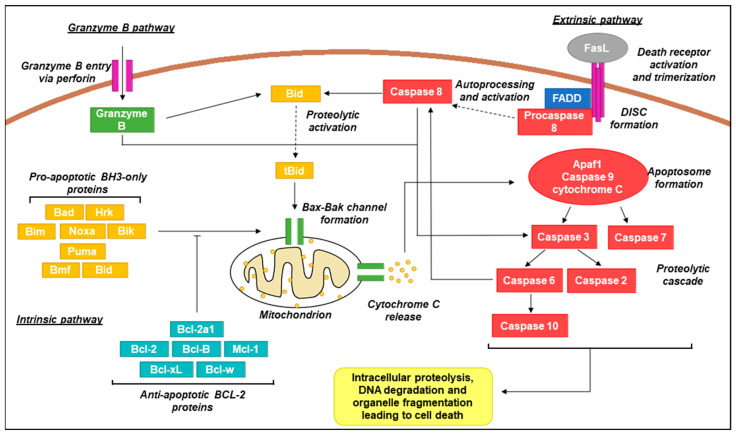
Figure 3.
Schematic representation of canonical apoptotic pathways. In the intrinsic pathway, pro-apoptotic BH3-only proteins act as sensors and interact with anti-apoptotic Bcl-2 proteins upon activation by stress signals. Activation over a critical threshold overcomes the anti-apoptotic effects of Bcl-2 proteins and promotes oligomerization of Bax/Bak channels in the mitochondrial membrane that permit the release of the intermembrane space protein cytochrome C [104]. Cytoplasmic cytochrome C promotes apoptosome formation (complex of Apaf1, caspase 9, and cytochrome C) which activates caspases 3 and 7. This results in a signalling cascade providing activation of additional caspase family members which proceed to act on a wide range of cellular targets, ultimately resulting in cell death [105,106]. In the extrinsic pathway, activation of death receptors (e.g., Fas, tumour necrosis factor receptor 1; TNFR1) by cognate ligands (e.g., Fas ligand;FasL, tumour necrosis factor alpha; TNF-α) recruits Fas-associated protein with death domain (FADD) adaptor proteins and procaspase 8 to form the death-inducing signalling complex (DISC) [107]. Procaspase 8 molecules aggregate resulting in autoprocessing and subsequent activation. Active caspase 8 activates caspase 3 and Bid (in its active truncated form; tBid) and converges with the intrinsic pathway via mitochondrial Bax-Bak channel formation [108]. In the granzyme B pathway, granules containing granzyme B and perforin are released from immune cells such as cytotoxic T lymphocytes and natural killer (NK) cells. Perforin oligomerises in the target cell membrane, allowing for entry of granzyme B which is also capable then of activating caspase 3 and Bid, similar to caspase 8 [109].