Figure 19.
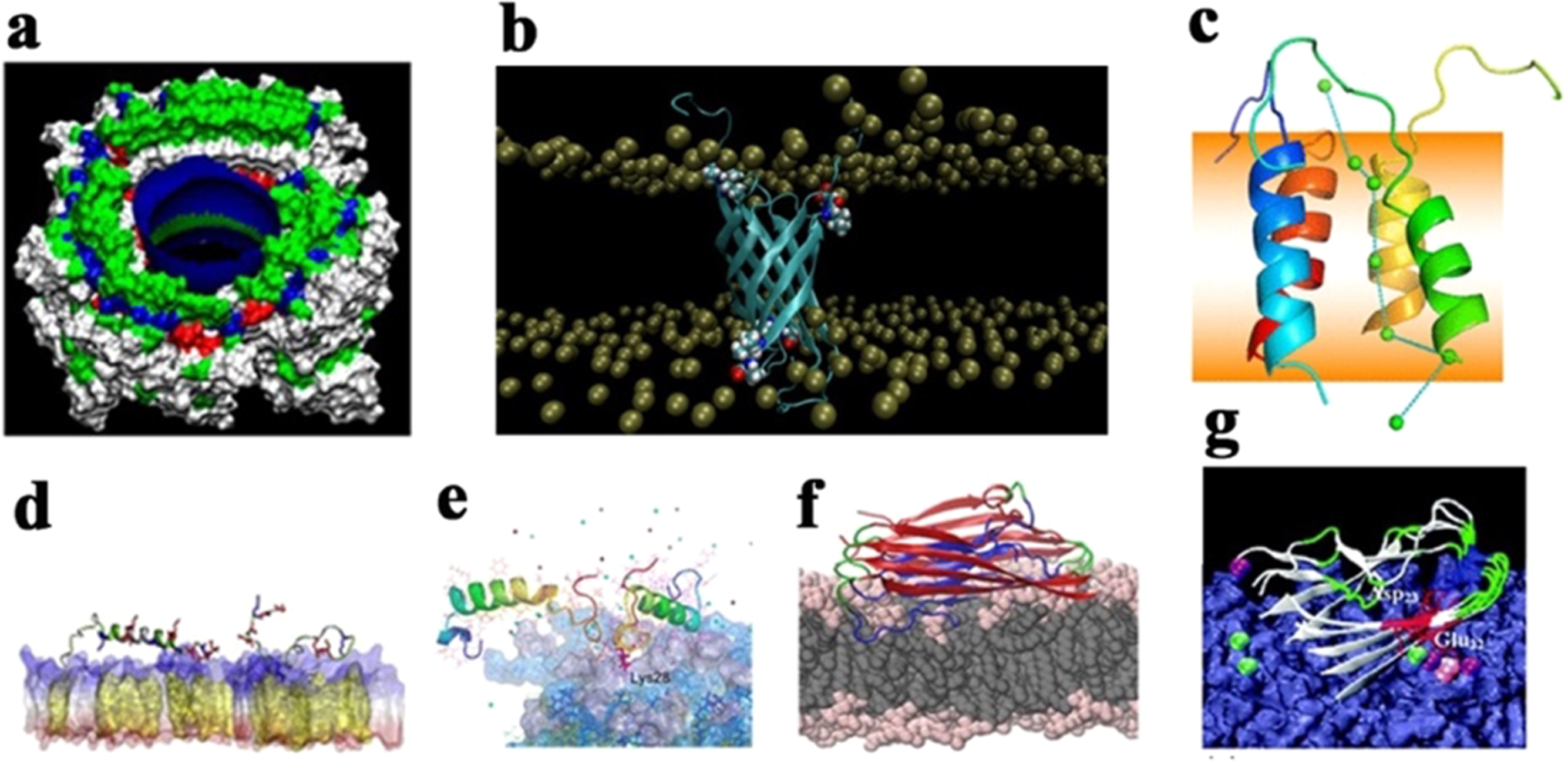
Different interaction models of full-length Aβ40/42 peptides with lipid membranes. (a) Aβ transmembrane pores with high Ca2+ permeability and selectivity.579 (b) Tetrameric Aβ42 β-barrel pores in PC/PS/cholesterol/sphingomyelin and DPPC bilayers.591 (c) Tetrameric Aβ42 α-helix-bundle pores in a DPPC bilayer with Ca2+ transport across the bilayer.596 (d) Aβ42 monomer with both α-helical and β-structure conformations being adsorbed on cholesterol-rich POPC bilayers, where increase of cholesterol promotes Aβ-membrane interactions and adsorption.603 (e) Aβ dimers on the GM1-clustering membrane, with C-terminal residues being inserted into the membrane.604 (f) Aβ tetramers with typical U-bent β-structure being preferentially adsorbed on and inserted into the POPE bilayer over the POPC bilayer, as driven by electrostatic interactions. (g) Aβ42 pentamer being adsorbed onto a POPC/POPG bilayer via Ca2+ ionic bridges between Glu22 and Asp23 and anionic headgroups of the lipid bilayer.610
