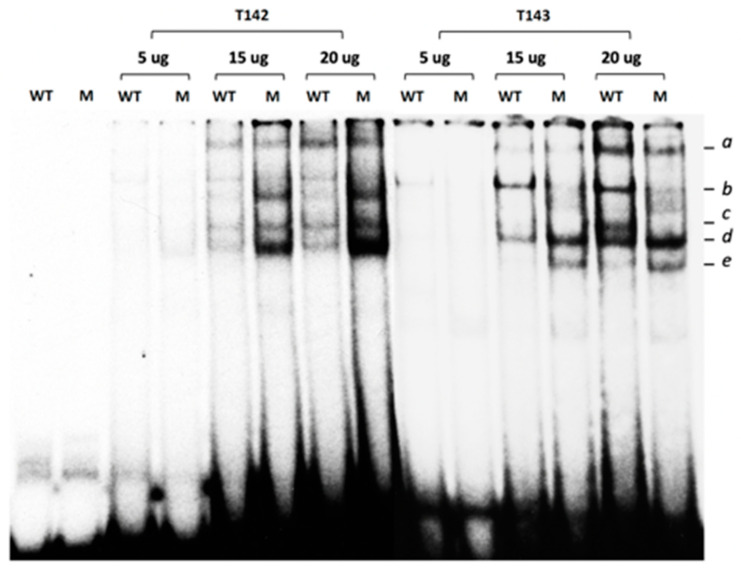
Figure 5.
Gel shift analysis of the nuclear proteins binding to the HTR2B −280 STAT site. Nuclear proteins (5-, 15- or 20 µg) obtained from the UM cell lines T142 and T143 were incubated with a 5′ end-labeled, double-stranded oligonucleotide bearing either the wild-type sequence of the −280 STAT site identified in the HTR2B gene promoter (WT), or a derivative in which the −280 STAT site was mutated (M). Formation of DNA-protein complexes was then monitored by EMSA. The position of the multiple DNA-protein complexes formed is indicated (a to e), along with that of the free probe (U). Incubation of increasing concentrations of T142 and T143 nuclear proteins with the WT −280 STAT labeled probe yielded the formation of distinct DNA-protein complexes of which only complex b completely disappeared when the wild-type probe was substituted by the mutated −280 STAT site (Mut).