Figure 3.
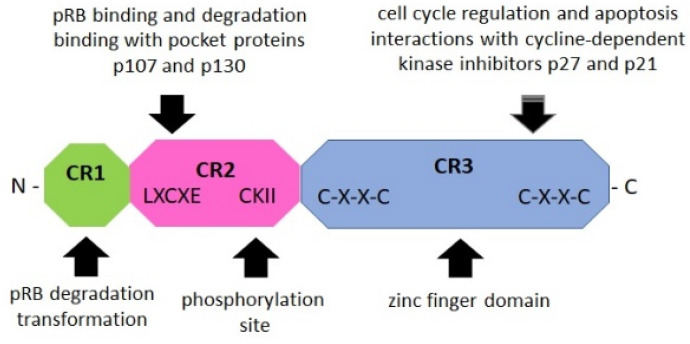
Schematic structure of the E7 oncoprotein (based on Boulet G. et al., 2007) [71]. The E7 oncoprotein contains three conserved regions (CR1/2/3). The NH2-terminal CR1 domain (green) is necessary for cellular transformation and pRB degradation but does not directly contribute to pRB binding. This domain interacts with host proteins such as E3 ubiquitin-protein ligase UBR4/p600 and p300/CBP-associated factor (PCAF), also known as K (lysine) acetyltransferase 2B [75]. The CR2 domain (pink) contains the pRB-binding core sequence LXCXE and a phosphorylation site for casein kinase II (CKII). The COOH-terminal CR3 domain (blue) is conserved and encodes a zinc finger domain containing two copies of the CXXC motif. This region is implicated in the association of pRB and other host cellular proteins. It is also critical for zinc-dependent dimerization and for mediating E7 interactions with cellular proteins crucial for cell cycle regulation and apoptosis (p21 and pRB) [10,71,75,76].