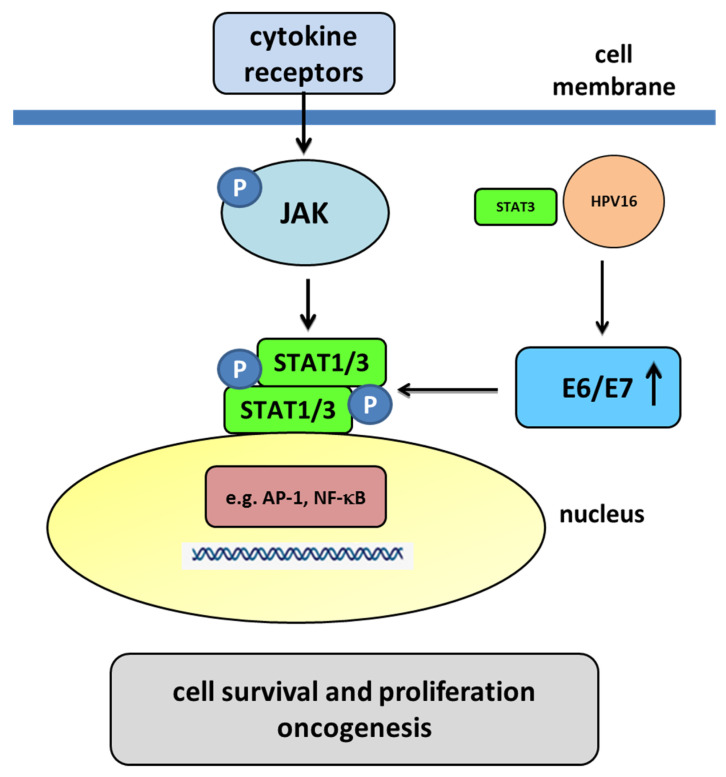
Figure 7.
The JAK/STAT pathway mediated by the E6 and E7 oncoproteins. Membrane cytokine receptors have cytoplasmic tails in which inactive JAKs associate constitutively. The cytokine interaction with their receptors induces dimerization of these receptors. Interaction between the cytokine and its receptor results in the juxtaposition of JAKs, leading to their autophosphorylation. The activated JAKs then phosphorylate the receptor’s cytoplasmic tails on tyrosine residues, creating sites that allow the binding of other signaling molecules, such as STAT proteins. Cytoplasmic STATs bind to phosphorylated receptors, becoming substrates for the JAKs, which phosphorylate STATs on highly conserved tyrosine residues. After their phosphorylation, the STATs form homodimers or heterodimers that are capable of translocating to the nucleus and activating gene transcription. The E6/E7 oncoproteins decrease the translocation of STAT1 to the nucleus. A decrease in STAT1 is necessary for the amplification of the viral genome in the early stages of infection, meaning that STAT1 plays a protective role in the early phase of HPV infection. In the nucleus, transcription factors such as AP-1 and NF-κB, as well as STAT3 may play a regulatory role in HPV infection. HPV-infected cells produce large amounts of IL-6 for autocrine signaling and for increasing STAT3 activation. Some studies have suggested that STAT3 could bind to HPV16 upstream of the URR, driving the expression of E6/E7. Activated STAT3 results in an increase in the E6 and E7 oncoproteins. The oncoproteins promote a decrease in pRB and p53, which are the proteins that are responsible for the inhibition and arrest of the cell cycle and the promotion of apoptosis. HPV16 oncogenes downregulate the expression of IFN-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes [121,130].