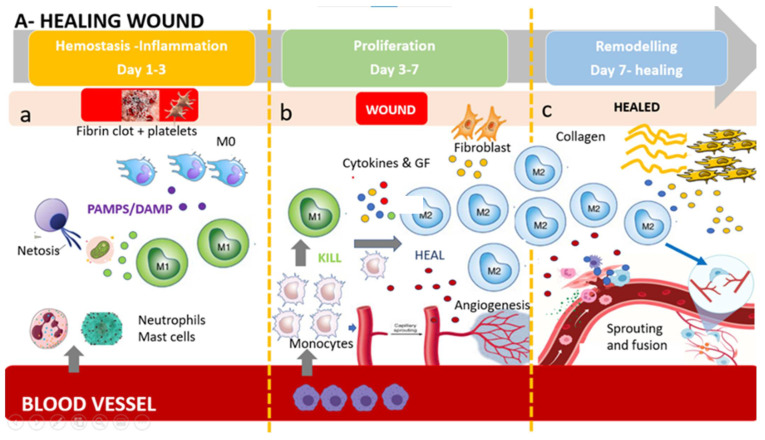
Figure 1.
The healing wound: (a) Platelets form a fibrin clot, and chemo-attractants are released to recruit inflammatory cells (neutrophils and mast cells), releasing pro-inflammatory cytokines. NETosis (Neutrophil Extracellular Trap) helps to capture and destroy pathogens. Tissue-resident macrophages react to pathogen- and damage-associated molecular patterns (PAMPs and DAMPs). First wave of monocytes differentiates in M1(phagocytoses step). (b) After the resolution of inflammation, the proliferative phase starts. Angiogenesis develops via vessel sprouting. Infiltrating monocytes differentiate into M1 and M2 macrophage subsets. M1 macrophages maintain a strong inflammatory profile releasing inflammatory cytokines and ROS and eating dead bacteria and neutrophils. After M1 polarize in M2 pro-regenerative phenotype which release anti-inflammatory cytokines, growth factors, and proteases which replace the provisional ECM with collagens, induce fibroblasts proliferation, and induce new vessel formation. This process results in granular tissue and keratinocyte coverage. (c) Remodeling is supported by macrophages, fibroblasts, and myofibroblasts re-organizing the provisional ECM into a definitive healed tissue, principally through matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), resulting in tissue with strong tensile strength and functionality. Angiogenesis is almost complete, and macrophages produce molecule bypass (fusion) between newly formed vessels, creating a functional network.