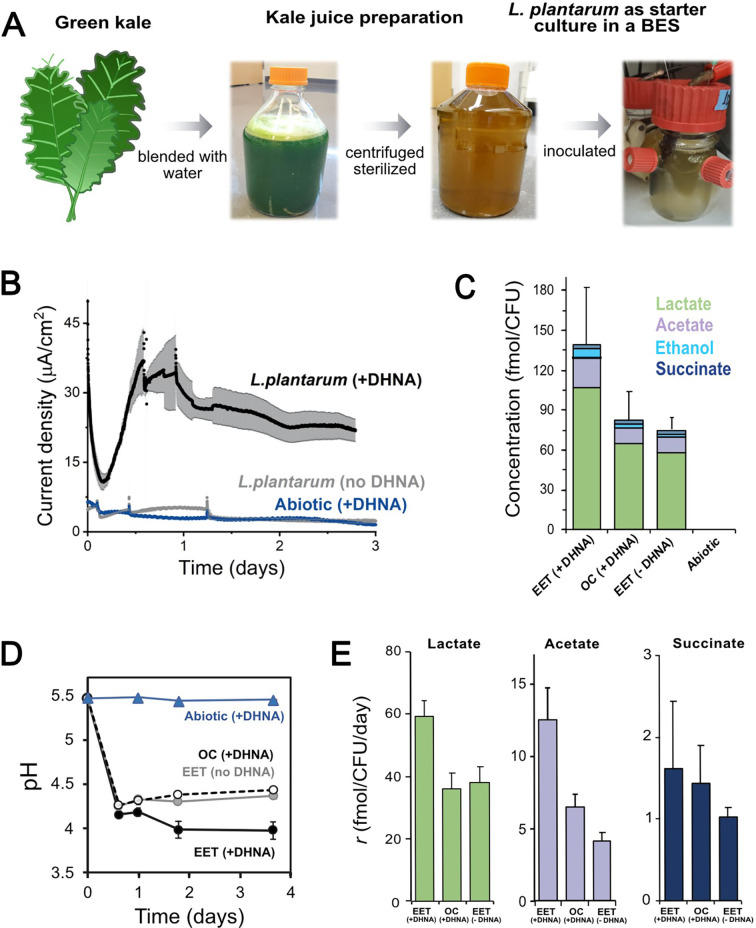
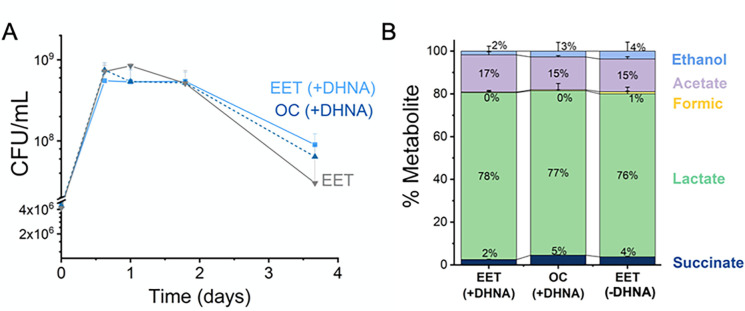
Figure 6. EET in a kale juice increases the production of fermentation end products.
(A) Preparation of kale juice medium used for fermentation in bioelectrochemical reactors. (B) Current density production measured from kale juice medium over time in the presence of L. plantarum and 20 μg/mL DHNA, no DHNA, or under abiotic conditions with addition of 20 μg/mL DHNA. The anode polarization was maintained at 0.2 VAg/AgCl. (C) Normalized total quantities of the metabolites detected per cell (CFUmax used for calculations). (D) pH measurements over time under different conditions tested on a second set of kale juice fermentations performed under the same conditions. (E) Production rate per viable cell, r, of lactate, acetate, and succinate. The avg ± stdev of three biological replicates is shown. See also Figure 6—figure supplement 1 and related data in Figure 6—source data 1.