Figure 7.
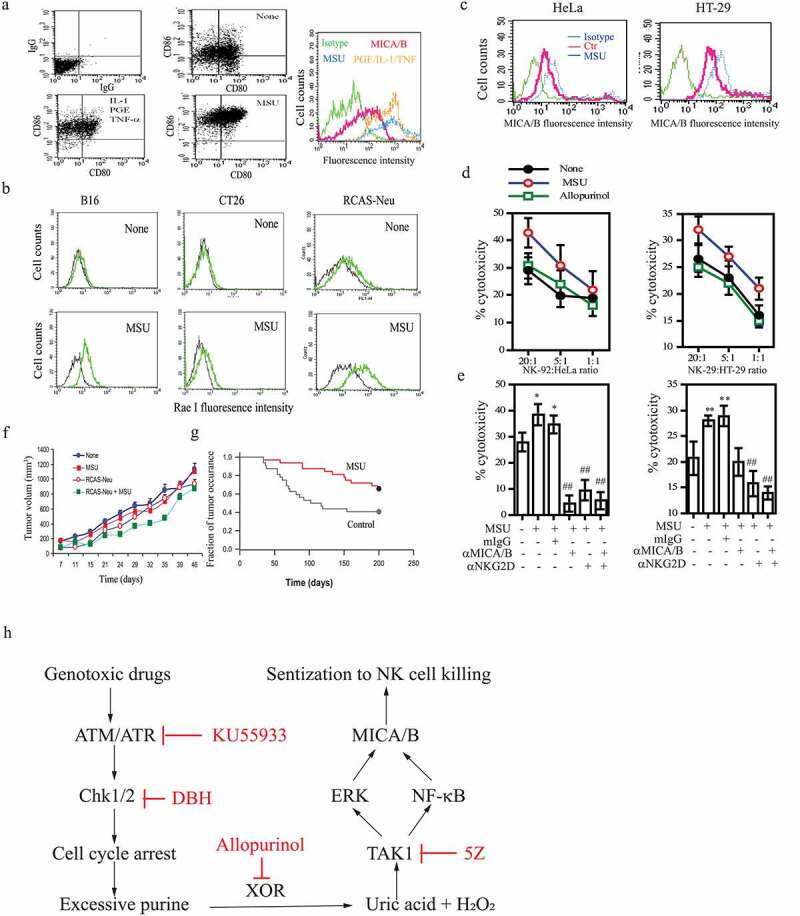
The in vitro and in vivo antitumor activity of MSU. (a) DCs were left unstimulated or stimulated with MSU (200 μg/ml) or a cocktail of the inflammatory cytokines (TNF-α, PGE2, and IL-1β) for 16 hr. Single-cell suspensions were analyzed for CD80 and CD86 expression by double staining with anti-CD80-FITC and anti-CD86-PE by FACS. Mouse IgG antibodies conjugated with PE or FITC were included as isotype controls. MICA/B expression was analyzed by staining with a FITC-conjugated anti-MICA/B antibody. Green line, isotype control; Red, Orange, and blue lines denote MICA/B in DCs left untreated or treated with the cytokine cocktail or MSU, respectively. (b) MSU induces NKG2D ligand expression in murine tumor cell lines. B16-F10, CT26, and RCAS-Neu cells were incubated with MSU (200 µg/ml) for 20 hr, single-cell suspensions were analyzed for Rae I expression by flow cytometry. (c) HeLa and HT-29 cells were pre-incubated with MSU (200 µg/ml) for 20 hr, the cells were harvested and analyzed for MICA/B expression by flow cytometry. (d) MSU-treated cells (20,000 cells/sample) were labeled with 51Cr for cytotoxicity assay and were mixed with NK-92 cells with the indicated ratios. After incubation for 4 hr, the supernatants were collected and assayed for 51Cr release. (e) HeLa and HT-29 cells were pretreated with MSU for 20 hr and then labeled with 51Cr. Tumor cells (20,000 cells/sample) and NK-92 cells (1x106/sample) were preincubated at 37°C for 30 min with an anti-MICA/B mAb (6D4) and anti-NKG2D mAb (clone 1D11), respectively. Mouse IgG was used as a negative control. Cells were mixed at the effector:target ratio of 1:10 and then incubated for 4 hr. The supernatants were collected and analyzed for the release of 51Cr in a scintillation counter. Data are the mean ± SD of three independent experiments. *p < .05, **p < .01, compared to the untreated control. ##p < .01, compared to mIgG control treatment. (f) Uric acid induces the antitumor immunity. RCAS-Neu cells were injected into the fat pad of FVB mice (6 mice/group) pre-immunized with MSU and/or irradiated RCAS-Neu. Tumor volumes were monitored twice weekly and plotted. (g) TVA transgenic mice infected with RCAS-Neu virus (1x106 virion/mouse) by intraductal injection were treated with saline or MSU. Mice were monitored for tumor occurrence for 7 months after tumor induction by palpation. Fraction of tumor-free mammary were plotted. MSU versus control, p < .01. (h) The schematic model of DNA damage-induced NKG2D ligand expression. Activation of the ATM-Chk pathway leads to cell cycle arrest. Excessive purine nucleotides are catalyzed by XOR to produce ROS and uric acid. The latter binds to and activates TAK1, a serine/threonine kinase that activates NK-κB and the MAP kinase pathway. Activation of NF-κB and the MAP kinase-activated AP1 transcription factor leads to transcriptional up-regulation of NKG2D ligand gene expression
