Summary
Immune responses must be rapid, tightly orchestrated, and tailored to the encountered stimulus. Lymphatic vessels facilitate this process by continuously collecting immunological information (i.e., antigens, immune cells, and soluble mediators) about the current state of peripheral tissues, and transporting these via the lymph across the lymphatic system. Lymph nodes (LNs), which are critical meeting points for innate and adaptive immune cells, are strategically located along the lymphatic network to intercept this information. Within LNs, immune cells are spatially organized, allowing them to efficiently respond to information delivered by the lymph, and to either promote immune homeostasis or mount protective immune responses. These responses involve the activation and functional cooperation of multiple distinct cell types, and are tailored to the specific inflammatory conditions. The natural patterns of lymph flow can also generate spatial gradients of antigens and agonists within draining LNs, which can in turn further regulate innate cell function and localization, as well as the downstream generation of adaptive immunity. In this review, we explore how information transmitted by the lymph shapes the spatiotemporal organization of innate and adaptive immune responses in LNs, with particular focus on steady state and Type-I vs. Type-II inflammation.
Keywords: In Vivo Imaging, Lymph Nodes, Inflammation, Dendritic Cells, Monocytes/Macrophages, T cells
Role of lymph and lymph nodes in the immune response
The primary function of the vertebrate cardiovascular system is the regulation of body homeostasis. Blood vasculature supplies oxygen and nutrients to cells and tissues as well as removes waste products for elimination, while the lymphatic system maintains tissue balance by returning interstitial fluids via lymph back into the circulation1. In addition to these essential functions, the cardiovascular system is also integrally connected to the organization and function of the immune system2,3. Most adaptive lymphocytes and many innate cell types continuously recirculate via the blood, and this promotes rapid trafficking to lymphoid and non-lymphoid organs for surveillance and host defense. The lymphatics, on the other hand, carry information pertaining to the state of peripheral tissues, such as antigens, immune cells, inflammatory mediators, and other soluble molecules2–7. The lymphatic vasculature originates within the tissues as a network of blind-ended, thin-walled lymphatic capillaries, which are highly permeable and specialized in fluid uptake1,3,8. The lymphatic capillaries within the tissues coalesce into larger collecting lymphatic vessels that facilitate lymph transport, and these vessels eventually converge onto the thoracic duct, which empties the lymph into the subclavian veins to return this fluid back into the blood circulation1.
Located along the path of lymph return are specialized lymphoid organs, called lymph nodes (LNs), which coordinate the information derived from adjacent peripheral tissues via the lymphatics, as well as promote surveillance of this information by the recirculating lymphocytes. Indeed, the formation of LNs themselves is intimately tied to both the blood and lymphatic vascular networks, as blood vessels supply the first lymphoid tissue inducer cells to drive early LN anlagen, while interstitial flow within the lymphatic vessels provides the spatial and mechanical cues to form the subcapsular sinus and promote LN expansion9. Critically, LNs are strategically positioned along the lymphatic network to intercept information transported by the lymph, in order to contain pathogens and limit dissemination, as well as to present this antigenic information to the recirculating T and B cells10. Lymph-borne proteins are further concentrated within LNs via the reticular conduit network, which transports fluids and small molecules back into the blood circulation, while retaining larger particulate proteins or pathogens for sampling by the LN innate sentinel cells11–14. These innate cells are localized in a highly organized manner within LNs, and this allows for an extraordinary level of efficiency in sampling of antigens, detection of dangerous insults and pathogens, transmission of signals among responding innate immune cells and cognate lymphocytes, and the generation of robust antigen-specific adaptive responses specifically tailored to the nature of the stimulus15–17 (Figure 1). Together, this intersection of information gathering via the lymphatic system, adaptive lymphocyte recirculation via blood vessels, and local cellular organization makes LNs ideal hubs for the induction and regulation of immunity, based on the specific organismal needs.
Figure 1. Initiation and organization of immune responses within lymph nodes.
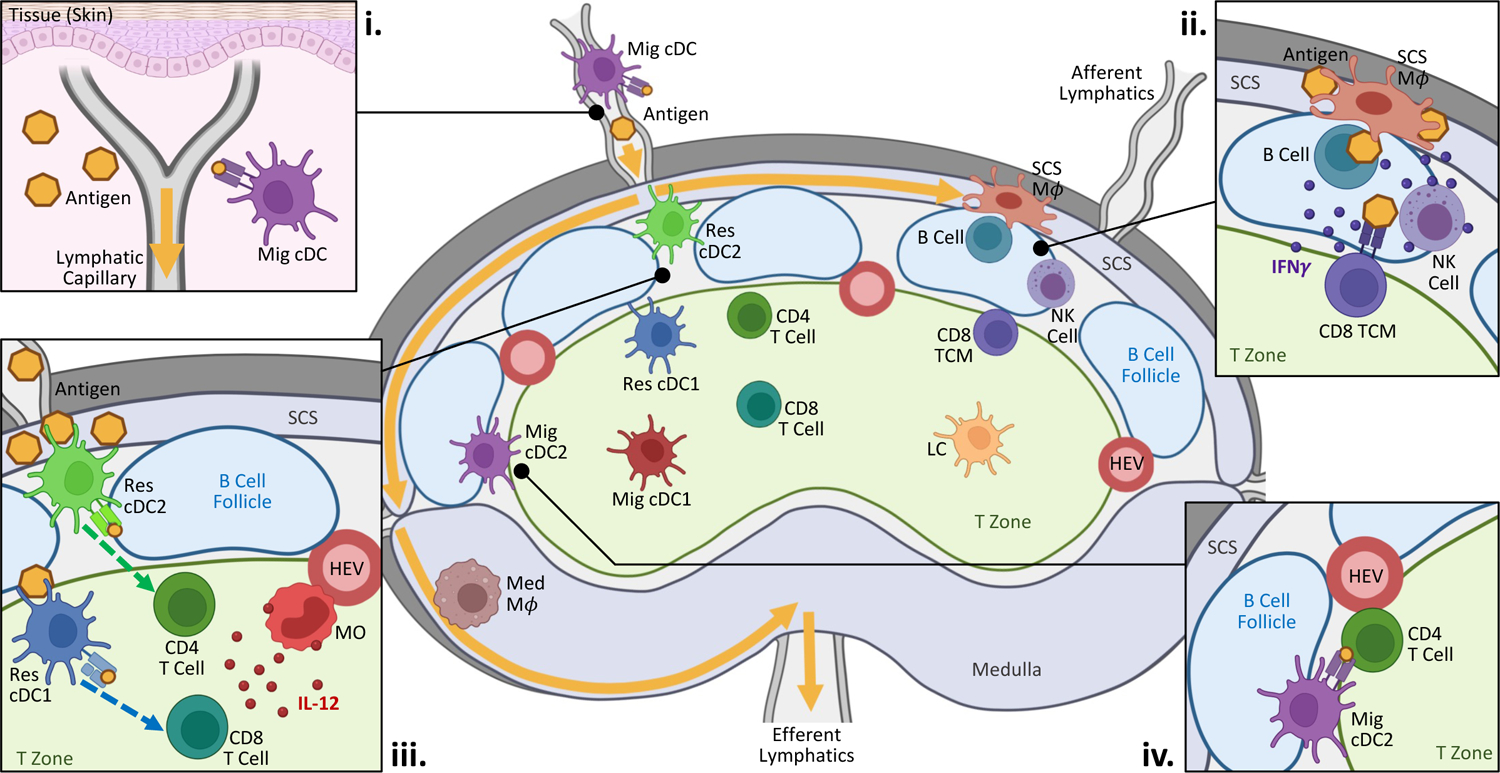
Diagram depicts the preferential localization of different immune populations within steady state LNs. Biased spatial positioning is seen for both innate and adaptive cell subsets. (i) At both steady state and during inflammation, information about the state of peripheral tissues is delivered into the nearest LNs via the lymphatics either through direct antigen drainage or active transport by migratory cDCs. (ii & iii) During Type-I inflammation, macrophages and resident cDCs located directly within or proximally to the lymphatic sinuses readily capture antigens and inflammatory agonists. (ii) Innate and adaptive immune cells positioned near the subcapsular sinus initiate early inflammatory responses to promote pathogen containment and clearance. (iii) Resident cDCs also undergo maturation and relocalize into the T cell zone, while inflammatory monocytes infiltrate the LN from the blood. Together, these two cell types functionally cooperate to drive early T cell activation and effector differentiation. Migratory cDC1s can also support Th1 differentiation. (iv) Antigen-bearing migratory cDC2s preferentially position along the T-B border, where they interact with responding CD4 T cells to promote TfH responses. During Type-II inflammation, these cDCs are also the dominant drivers of Th2 differentiation (also see Figure 2). Yellow arrows indicate direction of lymph flow.
Abbreviations: CD8 TCM, central memory CD8 T cell; HEV, high endothelial venule; LC, Langerhans cell; Mϕ, macrophage; Med, medullary; Mig, migratory; MO, monocyte; Res, resident; SCS, subcapsular sinus
Created with BioRenderer.com
Lymph node organization and function at steady state
At both steady state and during inflammation, lymph formed in upstream tissues enters LNs via the afferent lymphatic vessels, passes through the subcapsular and cortical lymphatic sinuses, and then exits via the efferent lymphatic vessels located in the LN medulla to eventually return to the blood circulation. Most LN macrophages, including subcapsular sinus macrophages lining the B cell follicles as well as medullary macrophages, are localized directly within the lymphatic sinuses (Figure 1). This enables efficient surveillance of draining lymph, and promotes rapid capture of pathogens that arrive via the lymphatics18,19. Strategically prepositioned close to the subcapsular sinus macrophages are multiple innate lymphoid cells, including NK cells, gamma-delta T cells, NKT cells, and innate-like CD8 T cells20–22. These cells are poised to immediately respond to macrophage-derived signals upon inflammation and help initiate early immune responses21. Central memory CD8 T cells are also localized nearby, and survey neighboring innate cells for cognate antigens presented on MHC molecules23–25. Together, these cells serve to patrol the outer periphery of the LN and defend the site against invading pathogens that disseminate from the lymphatics10.
In addition, our quantitative 2-dimensional (2D) and 3D imaging studies have revealed that LN resident conventional dendritic cells (cDCs), and in particular resident cDC2s, also preferentially localize near or directly within the lymphatic sinuses26,27. This enables robust sampling of draining antigens and allows these cells to induce T cell responses during inflammation13,27,28. While some resident cDC1s also reside within these lymphatic sinus regions, most cDC1s are found more evenly distributed throughout the T cell zone and in close association with LN blood vessels26,29. Although this reduces their efficiency in sampling of lymph-borne antigens13,27, more centralized positioning may enhance information exchange with migratory cDCs, which localize there, and allow the interception of potentially infected or dying cells during viral infections and cancer30–32. Close associations of resident cDC1s with blood vessels may also promote homeostatic maintenance of high endothelial venules to support normal lymphocyte recirculation29,33. These differences in cDC subset positioning have been recapitulated in human LNs, thus revealing the conservation of cDC subset patterning across species and suggesting likely functional importance for maintenance of homeostasis or host defense34.
cDCs residing directly within peripheral tissues also constitutively sample local antigens. In the steady state, tonic signaling within tissues induces a fraction of these cells to undergo homeostatic maturation35–38, and this induces their migration via the lymphatics into the nearest LNs in a CCR7-dependent manner6,39–41. Within LNs, different migratory cDC subsets segregate into distinct spatial compartments, and this is observed both at steady state and during inflammation16. Specifically, work by our group and others on cutaneous LNs (the focus of this review) has demonstrated that migratory cDC1s and Langerhans cells (ontogenically closely related to macrophages) preferentially migrate into the deep T cell zone, while migratory cDC2s generally localize to the interfollicular regions and outer LN paracortex26,29,42,43. Further segregation of migratory cDC2 subpopulations is seen based on CD301b and SIPRα marker expression. CD301b+ cDC2s are primarily found at the upper paracortex bordering the B cell follicles, while SIRPα- migratory cDC2s (also described as “triple negative” cDC2s) predominantly accumulate in the lower cortical ridge bordering the medulla29,44–46.
At steady state, lymph supplies a large repertoire of tissue-derived self-antigens, including byproducts from apoptotic cells and catabolized extracellular matrix components4,7. The dominant role of these steady state cDC populations in LNs is to present captured peripheral self-antigens on MHC molecules to T cells in a non-immunogenic fashion. This is essential for inducing peripheral tolerance to potential self-reactive T cells, which may have escaped central thymic selection and entered the circulation35,37,38,47. While cDCs are critical for this process, we have found that they can also aberrantly activate self-reactive T cells48,49. In these instances, local regulatory T cells help maintain immune quiescence by forming discrete clusters around the autoreactive cells and quickly constraining self-reactive responses48–52.
Stromal cells in the organization of lymph node innate immune cells
Such intricate compartmentalization of immune cells in LNs is thought to be at least in part mediated by the signals provided by the underlying stroma. Recent single-cell RNA sequencing studies have revealed the existence of multiple subsets of LN stromal cells with divergent properties, such as expression of distinct chemokines and adhesion molecules, as well as preferential production of survival factors to support immune cell homeostasis53–59. In brief, lymphatic endothelial cells (LECs) and marginal reticular cells line the subcapsular sinus and promote the homeostatic maintenance of subcapsular sinus macrophages60,61, as well as express chemokine scavenging receptors to establish CCL21 chemokine gradients that help guide migratory cDCs from the sinuses into the LN parenchyma62. Follicular dendritic cells, located within the B cell follicles, secrete abundant amounts of CXCL13 to recruit and position CXCR5+ naïve B cells, T follicular helper (TfH) cells, as well as CXCR5-expressing migratory cDCs in certain inflammatory settings58,63–66. In the paracortex, multiple populations of T-zone reticular cells (TRCs) can be found, which secrete the chemokines CCL19 and CCL21 to promote homing of CCR7-expressing T cells and activated cDCs to the deep T cell zone63,67–70. A unique population of CCL19-low TRCs are found along the T-B border and the interfollicular regions, and these stromal cells preferentially generate the chemotactic ligands for Ebi2 receptor-expressing immune cells54.
With regard to innate cell positioning, Ebi2 is preferentially expressed on resident and migratory cDC2 populations and is thought to promote their localization and maintenance within the marginal zone bridging channels of the spleen, as well as within the outer paracortical regions of the T cell zone71–73. Nevertheless, our quantitative imaging studies have demonstrated that disruption of the Ebi2 chemotactic axis has minimal effects on cDC distribution within cutaneous LNs74, suggesting that additional mechanisms can contribute72. Our group’s findings on the preferential localization of resident cDC1s, and to a lesser degree cDC2s, near LN blood vessels also suggest existence of additional guidance cues emanating either directly from the blood endothelial cells or the surrounding pericytes29. Of note, the discrete spatial patterning of resident cDC1 and cDC2 populations results in preferential coating of distinct vascular trees, with little intermixing between subsets, and this could be related to the substantial heterogeneity seen in the LN blood endothelial cells75,76. Alternatively, preferential cDC coating of vasculature may also result from local recruitment of pre-cDC1 vs. pre-cDC2 precursors via distinct blood vessels and their spatially segregated proliferation to repopulate the mature cDC subsets77–79. In this regard, a recently identified subset of Grem1+ TRCs has been shown to localize within the outer LN paracortex, a region highly enriched in high endothelial venules57,80. This stromal cell population expresses several genes related to cDC recruitment and survival, and plays a crucial role in resident cDC homeostasis. It must also be noted that reciprocal crosstalk of cDCs with both LN fibroblastic reticular cells and blood endothelial cells via lymphotoxin beta and Clec2a signaling has been shown to be critical for promoting the survival and function of LN stromal cells themselves, during both steady state and inflammation33,81–85. Thus, while the exact mechanisms remain to be worked out, the extensive stromal cell heterogeneity within LNs is thought to establish the appropriate spatial patterning and survival of various cDC subsets, and in return these cDCs support normal stromal cell homeostasis.
Generation of immune responses - Type-I Inflammation
In contrast to steady state conditions, inflammation of peripheral tissues, and specifically for this review, the murine skin, induces large-scale activation of immune cells both within the affected tissues and the draining LNs to support critical immunological functions. Many microbial infections of barrier surfaces or injected vaccines result in rapid release of pathogens, pathogen or danger associated molecular patterns (PAMPs, DAMPs), foreign antigens, alarmins, and other inflammatory mediators into the tissue parenchyma and subsequently the lymph3. Within minutes, these agonists flood the draining node, and the innate immune cells positioned within the lymphatic sinuses provide the first line of defense to contain the incoming threats as well as to trigger the initial wave of inflammation (Figure 1). Macrophages lining the lymphatic sinuses serve as local “flypaper,” and readily capture antigens, immune complexes, and pathogens as they enter through the afferent lymph. Medullary macrophages are highly phagocytic and rapidly degrade the captured microbes18. In contrast, subcapsular sinus macrophages are less degradative, and instead deliver the sequestered antigens to adjacent B cells to drive humoral immune responses86–89, as well as secrete type-I interferon (IFN-I) to alert and protect neighboring cells from further pathogen spread90–92.
In addition to direct containment, inflammasome activation and locally released cytokines from subcapsular macrophages elicits robust antigen-independent production of Type-II interferon (IFN gamma, IFNγ) by nearby innate lymphoid cells (NK cells, gamma-delta T cells, NK T cells, and innate-like CD8 T cells). This rapid wave of IFNγ in turn enhances the phagocytic activity of macrophages to amplify host defense21. Inflammasome activation also stimulates the influx of additional innate effector cells from the blood21,93, including neutrophils, which provide further support for pathogen containment21,94–96. Memory CD8 T cells close to the subcapsular sinus similarly secrete IFNγ upon detection of cognate antigen, inducing local CXCL9 production by macrophages and stromal cells. This leads to the repositioning of additional CXCR3-expressing memory T cells towards the site of infection, and these bystander-activated CD8 T cells rapidly mediate pathogen clearance23,24,97. CXCL9 can also be displayed on the lumen of high endothelial venules, enabling additional recruitment of central and effector memory CD8 T cells from the circulation98. Thus, within just a few hours of inflammation, various innate effector and adaptive memory cells that are pre-positioned in or rapidly recruited to the subcapsular sinus niche create a barrier to pathogen entry and prevent dissemination into the blood circulation as well as the deeper parenchymal regions of the LN.
Also enriched near the lymphatic sinuses are LN resident cDCs, and as shown by our group as well as by others, this allows them to efficiently intercept and present draining antigens27,28,36,99,100. The peripheral positioning of resident cDCs near the lymphatic sinuses, however, minimizes their chances of interacting with cognate naïve T cells, which are primarily localized within the T cell zone. In contrast to macrophages, which recruit immune cells locally, we demonstrated that activated resident cDCs instead undergo rapid maturation to express the chemotactic receptor CCR7, and use the CCL19/CCL21 chemotactic gradient within the LN parenchyma to relocalize from the lymphatic-rich peripheral regions into the T cell zone28. As these resident cDCs mature, they also increase surface expression of MHC and costimulatory molecules, thus facilitating enhanced antigen presentation and subsequent scanning by and activation of cognate T cells28. Further efficiency in reducing the search time for cognate cDC–T cell interactions is achieved directly within the T cell zone. As we and others have shown, resident and migratory cDC subsets segregate here, with activated cDC2s preferentially localizing in the outer paracortex, and cDC1s predominantly positioned within the deeper T cell zone, and this is conserved across most, but not all, Type-I inflammatory conditions16,28,72,73. This segregation of innate cells is also mirrored by the non-random distribution of naïve CD4 and CD8 T cells. CD4 T cells express higher levels of Ebi2 and also preferentially localize within the outer paracortex, thus paralleling the distribution of Ebi2-expressing cDC2s74,101. Given the enhanced abilities of cDC2s in MHC-II antigen presentation, and the converse specialized ability of cDC1s to cross-present antigens on MHC-I, we found that this spatial coordination between the cDC and T cell subsets further reduces the search space necessary for the T cells to locate their MHC-presenting cognate antigens, and ensures rapid generation of T cell responses in the event of an inflammatory insult74.
Our group as well as others have also demonstrated that as the resident cDCs migrate towards the T cell zone to elicit these cognate responses, inflammatory monocytes within the blood circulation also begin to infiltrate the draining LNs in large numbers via the high endothelial venules28,102–104. Upon entering the LN parenchyma, the recruited monocytes acquire markers of activation and migrate into the T cell zone, eventually colocalizing with the co-clustered resident cDCs and early activated T cells28. In comparison with resident DCs, monocyte maturation and entry into the T cell zone is relatively delayed, and monocytes do not appear to play a significant role in early antigen presentation and T cell activation in these settings. However, monocytes do arrive at the optimal time to provide an ample source of IL-12 to support the differentiation of recently activated T cells, and thus cooperate with the cDCs to enhance T cell effector programming28,102,104. Therefore, we suggest that the spatiotemporal dynamics of resident cDC and monocyte migration, maturation, and function represent critical features of T cell activation and differentiation in LNs during Type-I inflammation.
Following these very early T cell activation events, additional involvement of the CXCL9/10–CXCR3 chemokine axis has been shown to help reinforce subsequent effector T cell programming105. Expression of CXCL9 and CXCL10 chemokines by stromal and myeloid cells in the peripheral regions of inflamed LNs, such as the interfollicular zones and along the T-B boundary, elicits the recruitment of early differentiated effector T cells expressing CXCR3103,106–110. Multiple cellular sources likely contribute to the generation of this chemotactic gradient, including monocytes which are significant producers of CXCL9 and CXCL10 following skin immunization103,106–109. Migration of early effector T cells towards these microenvironments exposes these cells to additional sources of antigen and inflammation, and thus further enhances their differentiation103,107,108. Of note, early activated CD4 T cells expressing Tbet and CXCR3 can also utilize this chemotactic axis to migrate to the T-B border and promote humoral immunity110. In contrast, less-differentiated CD8 T cells are predominantly retained within the deep T cell zone, which further enhances the bifurcation of T cell responses107.
Additional innate support for the generation of T cell responses during Type-I inflammation has been shown for NK cells and plasmacytoid DCs (pDCs). IFNγ secreted by NK cells within the subcapsular sinus regions can enhance the activation of neighboring myeloid cells. Consistent with this, depletion of NK cells or blockade of IFNγ in certain inflammatory settings can lead to reduced cDC and monocyte responses, including diminished expression of IL-12 and CXCL9106,111–113. Reciprocally, IL-12 or IL-18 cytokines released by the activated cDCs or monocytes may amplify NK cell-mediated IFNγ production to create a positive feed-forward inflammatory cascade109,112,114,115. pDCs have also been shown to facilitate adaptive responses. During viral infection, pDCs can undergo CCR5-mediated intranodal migration within LNs to colocalize with responding CD8 T cells and cDC1s in order to optimize cDC1 maturation and downstream T cell priming via IFN-I signaling116. The CCR5 chemotactic axis has also been previously shown to enhance CD4 T cell help to CD8 T cell responses, by promoting more efficient scanning of cognate antigens by naïve CD8 T cells and for generating productive memory responses30,31,117–121.
In addition, migratory cDCs residing directly within inflamed tissues also integrate pathogen-derived signals and inflammatory cues, undergo full maturation, and traffic to the draining LNs in a CCR7-dependent manner to facilitate the generation of adaptive immunity36,41,122. The relative contributions of migratory vs. resident cDCs in T cell priming appear to be condition specific. As we and other groups have demonstrated, in settings of robust antigen and inflammatory agonist drainage via the lymph, with the exception of TfH responses, migratory cDCs appear mostly dispensable for early T cell responses27,28,99,100,123. However, during highly tropic or slowly replicating microbial infections, such as HSV infection, foreign antigens and stimuli remain largely trapped in the periphery and do not enter the lymph in sufficient quantities to elicit responses by LN-resident innate cells. In these settings, antigen-bearing migratory cDCs are indispensable for the initiation of T cell immunity31,124,125. In particular, migratory cDC1s can produce copious amounts of IL-12 and directly support the induction of Th1 and CD8 effector responses124,126,127. Dermal cDC2s can also undergo functional maturation to support Th1, Th2 (below) or Th17 polarization, based on the specific types of encountered stimuli122,128,129. In addition to direct induction of T cell responses, in certain infection or tumor models, migratory cDCs can transfer antigens to resident cDCs to further potentiate T cell activation30–32,130. It has also been shown that lymph-borne antigens can be stored within LN LECs for extended periods of time, and then slowly released to the transmigrating migratory cDCs, leading to continued induction of T cell immunity131–133.
In sum, Type-I inflammation induces a large-scale, orchestrated activation, recruitment, and crosstalk of multiple innate and adaptive cell types within draining LNs, which together promote rapid pathogen containment, as well as facilitate optimal generation of adaptive immunity.
Generation of immune responses - Type-II Inflammation
In contrast to Type-I responses discussed above, Type-II inflammation of cutaneous tissues does not appear to elicit strong LN resident innate responses. As we and others have shown, this is characterized by the absence of resident cDC activation, lack of IFNγ production by innate cells, and minimal monocyte influx and IL-12 expression28,106,109. In fact, absence of such stimuli (i.e., IFNγ and IL-12) may be essential for promoting Th2 immunity, as these signals can readily override the program of Th2 differentiation134. Consistent with this, migratory cDC1s, which constitutively secrete IL-12 within LNs, can inhibit Th2 responses, while genetic ablation of cDC1s can enhance Th2 immunity and promote control of nematode infection of the intestine135, or increase house dust mite induced airway inflammation136.
Nevertheless, Type-II inflammation still requires critical innate–adaptive immune cell crosstalk and the generation of specialized microenvironments within LNs to support Th2 responses (Figure 2). In these settings, peripheral tissue-derived migratory cDC2s, and in particular the IRF4/Klf4-dependent and CD301b+ subsets, appear to play a dominant role in Th2 induction45,109,137–141. Exposure to inflammatory stimuli and alarmins following barrier tissue damage from proteases or helminths leads to a specific program of maturation in local dermal cDC2s, and their migration via the lymphatics to the LN subcapsular sinus44. Here, additional CCL8 secretion by activated subcapsular sinus macrophages can synergize with CCR7 chemotactic signaling to promote CD301b+ cDC2 entry into the LN parenchyma142. Once in the LN, these CD301b+ cDC2s appear to predominantly localize at the T-B border45,142. Given that we and others find similar spatial patterns in steady state LNs29,45, this indicates that such localization is largely intrinsic to the cDC subsets, rather than elicited by the specific inflammatory conditions. In accordance with migratory cDC2 localization, Th2 cells also appear to accumulate along the T-B border65,143,144, suggesting that cellular crosstalk between cDC2s and CD4 T cells within this LN microenvironment may be necessary for optimal Th2 generation. Indeed, in addition to Ebi2, preferential expression of CXCR5 by migratory cDC2s has been in part attributed to their enrichment along the T-B boundary, and this positioning was found critical for induction of Th2 responses during nematode intestinal infection65,145.
Figure 2. Distinct immunological programs involved in the initiation of Type-I vs. Type-II immunity.
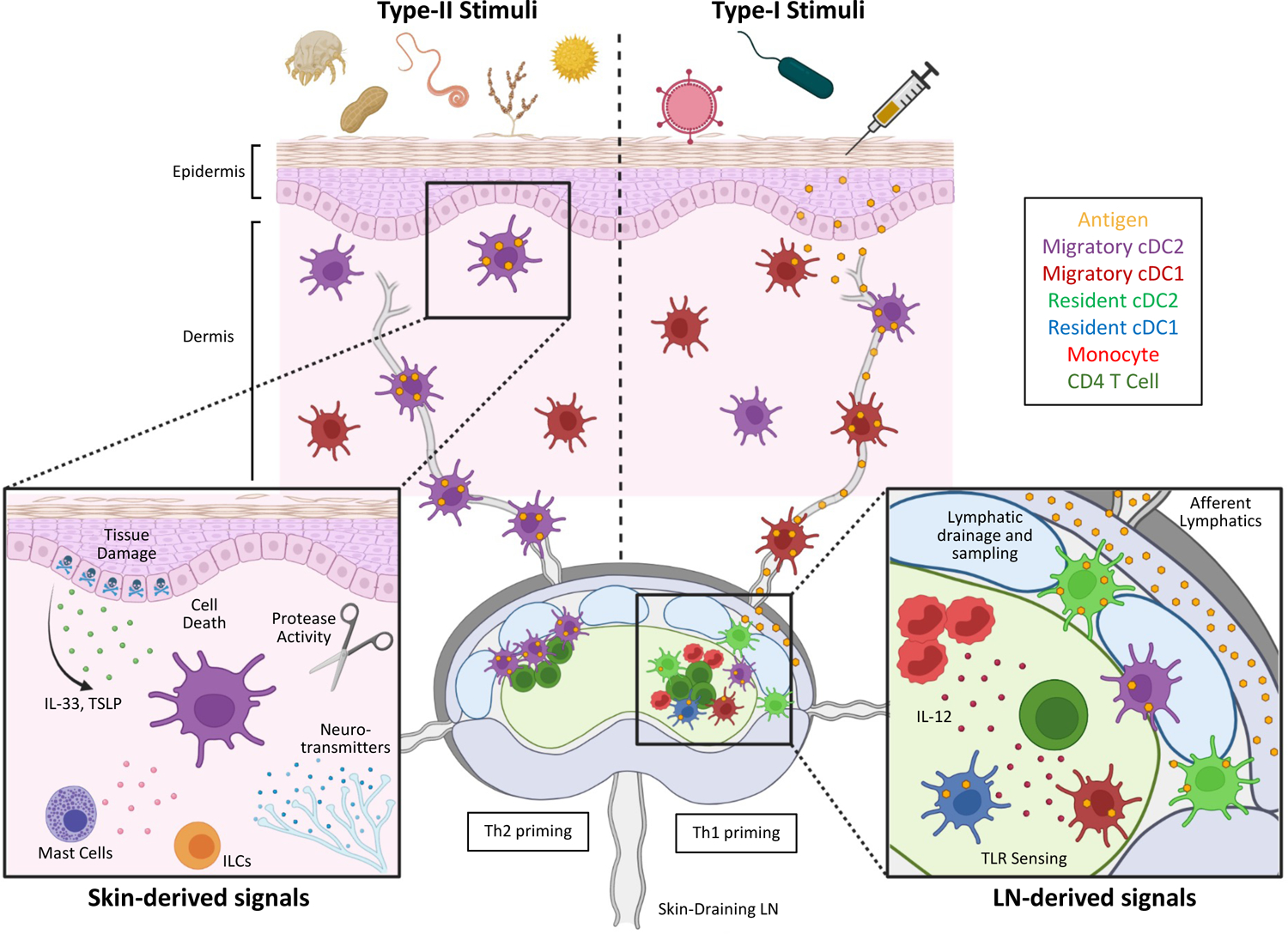
Type-II responses (left) are elicited in response to multiple skin-derived stimuli generated due to tissue damage and cell death. Various epithelial-derived alarmins, as well as mast cell-, neuronal-, and ILC-derived signals are integrated by the local dermal cDC2s, which next migrate into LNs to induce Th2 cell differentiation at the T/B border. In contrast, Type-I inflammatory settings (right) are elicited in response to innate sensing of microbe-associated molecular patterns and downstream inflammatory cytokines. In settings of ample antigen and agonist drainage to LNs, T cell responses are primarily induced through cooperation between LN-resident cDCs and blood-derived monocytes, as well as through participation of additional innate cell types (also see Figure 1). During highly tropic infections, peripheral tissue-derived dermal cDC1 and cDC2s more dominantly promote the generation of responses. Location of early activated Th1 and Th2 T cells depends on the positioning of specific antigen-presenting cDC subsets, as well as the chemokine receptors expressed on the responding cells.
Created with BioRenderer.com
Specific signals provided by migratory cDC2s to induce Th2 differentiation remain enigmatic122,141. Expression of OX40L and Notch ligands on cDCs have been shown important, but whether these molecules selectively promote Th2 responses or more broadly enhance the generation of multiple CD4 T cell helper subsets remains to be demonstrated146–149. IL-4 signaling has been long known to drive Gata3 expression and promote Th2 differentiation in vitro149. While the in vivo role of IL-4 in Th2 programming and localization has been less clear due to the confounding contributions of IL-4-producing TfH cells150,151, a more recent study has clarified that IL-4 is indeed critical for generating early Th2 responses in vivo152. Nevertheless, the very early cellular source of IL-4 to initiate the Th2 differentiation cascade in the LN has not been definitively established, and migratory cDC2s do not appear to express this cytokine. Instead, basophil recruitment to reactive LNs or local NKT cell activation within the interfollicular regions have been proposed, albeit the precise roles of these innate cells in Th2 differentiation have come under scrutiny153–157. It has recently been suggested that cDC-derived IL-10 signals can also elicit Gata3 expression and Th2 responses, indicating that multiple cytokines may mediate this process158. Given that several cell types can express IL-10 during inflammation, additional studies to explore the precise role of cDC-derived IL-10 in Th2 priming are necessary. Finally, TCR signal strength has also been shown to influence Th2 polarization both in vitro and in vivo, although how this contributes to polyclonal T cell responses in more complex in vivo settings of infection or protease-mediated tissue damage remains to be explored141,159–161. Thus, a combination of multiple stimuli provided by migratory cDC2s, and possibly other cells, as well as the complete absence of Th1 polarizing stimuli, within the peri-follicular microenvironments in LNs mediate the generation of Th2 responses.
It is important to highlight that in addition to Th2 differentiation, migratory cDC2s can also participate in the generation of multiple additional CD4 T helper cell subsets (Th1, Th17 and TfH), based on the specific nature of encountered agonist109,128. This indicates that migratory cDC2s are relatively plastic and can drive divergent T cell helper subtypes though distinct inducible transcriptional programs imparted on them by the signals encountered within the peripheral tissues, rather by their intrinsic propensities to promote one or another form of immunity. In particular, migratory cDC2s have been closely linked with the generation of TfH responses141,162–164. During infection, antigen-bearing migratory cDC2s accumulate at the T-B border45,65,163, and express the high affinity IL-2 receptor alpha chain (CD25). This helps augment Tfh differentiation by quenching T cell-derived IL-2 signals, which would otherwise restrict BCL6 expression in responding lymphocytes162. Similarly, during intestinal nematode infection, localization of cDC2s near B cell follicles via CXCR5 chemotactic signaling is critical for the induction of both Th2 and TfH responses65. This suggests that the close spatial juxtaposition of migratory cDC2-driven T cell responses with nearby B cell follicles may promote downstream interactions between activated T and B cells and thus elicit TfH programming64. In addition, during certain viral infections, early exposure to IFN-I leads to IL-6 production by migratory cDC2s, and this can help promote Tfh polarization164. Surprisingly, while most experimental models have demonstrated the combined loss of Th2 and TfH responses after broader blockade of cDC2 migration using conditional IRF4 deficiency models138,163, selective depletion of CD301b+ cDC2s resulted in enhanced generation of TfH responses. This suggests the potential for reciprocal cross-regulation of T cell differentiation by the different migratory cDC2 subsets165. Together these data support the model that migratory cDC2s are potent sensors and integrators of information within peripheral tissues and promote the generation of adaptive responses based on the corresponding organismal needs109.
Finally, additional migratory antigen presenting cells are also involved in generation of other helper cell responses in certain cutaneous infection settings. In particular, during Candida albicans fungal infection, Langerhans cells migrate to draining LNs and secrete polarizing cytokines, such as IL-1β, IL-6, and IL-23 to promote Th17 differentiation124,129. Given the preferential localization of these cells within the deeper regions of the T cell zone, as well as the unique cytokine profiles after fungal exposure, the LN microenvironments associated with Type-III inflammation are also likely to be different from other inflammatory settings.
Differences between Type-I and Type-II responses
These studies make it clear that divergent programs of T cell immunity (e.g., Type-I vs. -II) are driven by the distinct participation and activation states of specific innate immune cell subsets in the reactive LNs and the site of initial inflammation. It is therefore critical to consider how such innate responses are elicited in the different inflammatory conditions. In this regard, the mechanisms leading to the induction of innate Type-I vs. -II responses are quite distinct (Figure 2). As noted above, Type-I inflammation is initiated by the recognition of microbial and vaccine-derived PAMPs detected by conserved pattern recognition receptors on both innate and stromal populations36,122,141,166,167. This elicits a potent multicellular cascade of inflammatory signaling events, that together drive adaptive immunity. We propose that inflammatory signaling within both barrier sites and LNs likely contribute to functional adaptive responses, and the relative contribution of these is dictated by pathogen tropism, speed of replication, as well as the amount of cellular damage and local inflammation caused by the infection. In the case of vaccination, the formulation of antigen, type/size/charge of adjuvant, as well as the site of immunization will dictate the relative participation of tissue vs. LN -derived innate responses in driving T cell immunity168,169.
In contrast, Type-II responses are thought to be initiated by the recognition of “patterns of pathogenesis” in the form of allergens, venoms, adjuvants, cell death, tissue stress and destruction, and the release of non-specific alarm signals from barrier tissues, that together act on a variety of tissue-localized immune cell types170. Protease activity is likely one major upstream trigger of Type-II immunity, and most clinically relevant allergens have protease activity which can cause local activation and release of alarmins from the epithelial cells, as well as cleave epithelial tight junctions in affected barrier surfaces171–174. Helminth parasites, which drive potent Type-II responses, also employ proteases for migration through host tissues175,176, thereby causing widespread tissue damage. Other forms of cell death, such as migration-induced cell shattering of mononuclear phagocytes, have also been recently associated with Th2 differentiation177. Thus, the initiation of adaptive immunity during Type-II settings is likely to be critically dependent on innate cell sensing of local signals induced by tissue damage directly within the affected site.
Several immune cell populations reside in the skin, which together form complex cellular networks that function to maintain barrier integrity as well as promote immune responses. cDCs within these networks physically interact with and integrate signals from multiple distinct cell types before migrating into the nearest LN to instruct T cell responses122,178,179. With regards to Th2 generation, barrier tissue damage elicits the release of specific inflammatory mediators (i.e., IL-33, IL-18, IL-25, and TSLP), and these signals can act directly on locally pre-positioned cDCs, as well as several other nearby cell types, to induce their activation149,171,180. In particular, TSLP released by damaged epithelial cells activates cDCs within the skin, and this can drive the upregulation of OX40L and other costimulatory molecules, as well as promote their accumulation within draining LNs to induce Th2 responses in certain settings46,147,181,182. Similarly, IL-33 can also lead to the maturation and upregulation of co-stimulatory molecules in cDCs, suppress IL-12 expression, as well as mediate their migration to LNs183–186.
In addition, other responding immune cell types also potentiate cDC2 activation in the skin. Mast cells have been shown to directly interact with cDCs within the dermis187, and can release inflammatory mediators to induce the maturation and migration of neighboring cDCs to the skin-draining LNs179,187–189. Activated ILC2s can further amplify cDC responses through the production of the Type-II cytokine, IL-13190,191. In many tissues, including the skin, ILC2s predominantly reside within the adventitial cuffs, sites highly enriched with small blood vessels, lymphatics, peripheral nerves, as well as other key components of immunity192,193. Notably, CD301b+ cDC2s can colocalize with ILC2s within these niches192, suggesting that crosstalk between these two cell types may preferentially take place within this unique tissue microenvironment. More recently, a role for neuronal signaling has also been demonstrated in Type-II responses. Within the dermis, CD301b+ cDC2s can be found closely associated with the sensory neurons. TRPV1+ sensory neurons in particular can respond to allergens, and upon activation, locally release the neuropeptide Substance P to promote CD301b+ cDC2 migration into draining LNs and subsequent generation of Th2 responses194. Finally, an additional role for IFN-I signaling in cDC2-mediated Type-II immunity has also been noted44,195.
Thus, in contrast to anti-microbial Type-I responses which are characterized by broad-scale induction of innate cell activation through conserved microbial pattern recognition receptors and cytokine signaling in both peripheral tissues and draining LNs, Type-II responses appear highly dependent on the activation of multiple cell lineages within peripheral tissues in response to local tissue damage. Combined action of these cells appears to impart a unique differentiation trajectory for the locally distributed dermal cDC2 cells, which in turn migrate to draining LNs to elicit the corresponding Th2 responses. Together, this supports the model that the induction of Type-I vs. -II signatures of adaptive immunity are initiated as evolutionarily conserved mechanisms allowing either protection against microbial pathogenesis vs. wound repair and healing of tissue damage, respectively172.
Information gradients in the generation of immune responses
Additional considerations to how immune responses are propagated within reactive LNs are the spatial distribution and kinetics of lymph-borne antigens, inflammatory agonists, and the incoming migratory cDCs (Figure 3). Imaging studies by us and others have demonstrated that drainage of antigens and other soluble mediators from the inoculation site to LNs can happen within several minutes13,14,99,196–198. Upon reaching the lymphatic sinuses, proteins and particles are physically separated based on molecular weight, with smaller antigens (<70kDa) and fluids entering the LN conduit network distributed throughout the interfollicular regions and the T cell zone197,199–202. These conduits are also directly connected to the nearby blood vessels, and within a few hours, fluid-borne antigens, cytokines, and chemokines that entered the conduits are rapidly cleared out into the circulation. This can promote deposition of chemokines and inflammatory mediators on the lumen of high endothelial venules and lead to enhanced trafficking of immune cells into the reactive LNs200,203,204. In contrast, large antigens and pathogens are retained within the lymphatic sinuses, and instead can directly cross the floor of the lymphatic sinuses into the parenchyma either by passive diffusion via local fenestrations or by active transcytosis by the LECs13,14,196,205. We have previously shown that this results in enhanced accumulation of certain antigens within the lymphatic sinuses and the sinus-proximal parenchymal space, and the generation of steep antigen gradients across the LN. Significantly greater amounts of antigen are deposited near the lymphatic sinuses, while reduced quantities are found within the deeper T cell zone13. Given the preferential distribution of macrophages and resident cDC2s near the lymphatics, this leads to increased uptake of antigens by these populations13,14. In contrast, lower abundance of antigens within the deeper T cell zone leads to reduced antigen capture by the centrally localized cDC1s. As a consequence, subunit immunizations with limiting doses of antigen result in enhanced CD4 T cell activation as compared to CD8 T cells13. This suggests that the spatial positioning of cDC subsets within the LN, coupled with the generation of antigen gradients across the tissue, directly impacts which classes of cellular immunity are generated. Such spatial regulation of T cell responses may reflect the distinct roles of these T cell subsets in immunity. Preferential CD4 T cell responses to lymph-borne soluble antigens may promote an appropriate balance of activation against extracellular draining antigens. Early and efficient activation of CD4 helper T cells may also be necessary in order for these cells to provide timely help for CD8 T cell and humoral responses30,31,64,118,206. This is in contrast with intracellular infections, which are less likely to result in copious amounts of soluble antigens. In these settings, the more centrally localized cDC1s may be optimally positioned to acquire and cross-present antigen derived from infected host cells or antigen-bearing migratory cDCs, thus promoting robust CD8 T cell responses during conditions when the effector functions of cytotoxic CD8 T cells would be most utilized30–32.
Figure 3. Generation of information gradients within lymph nodes.
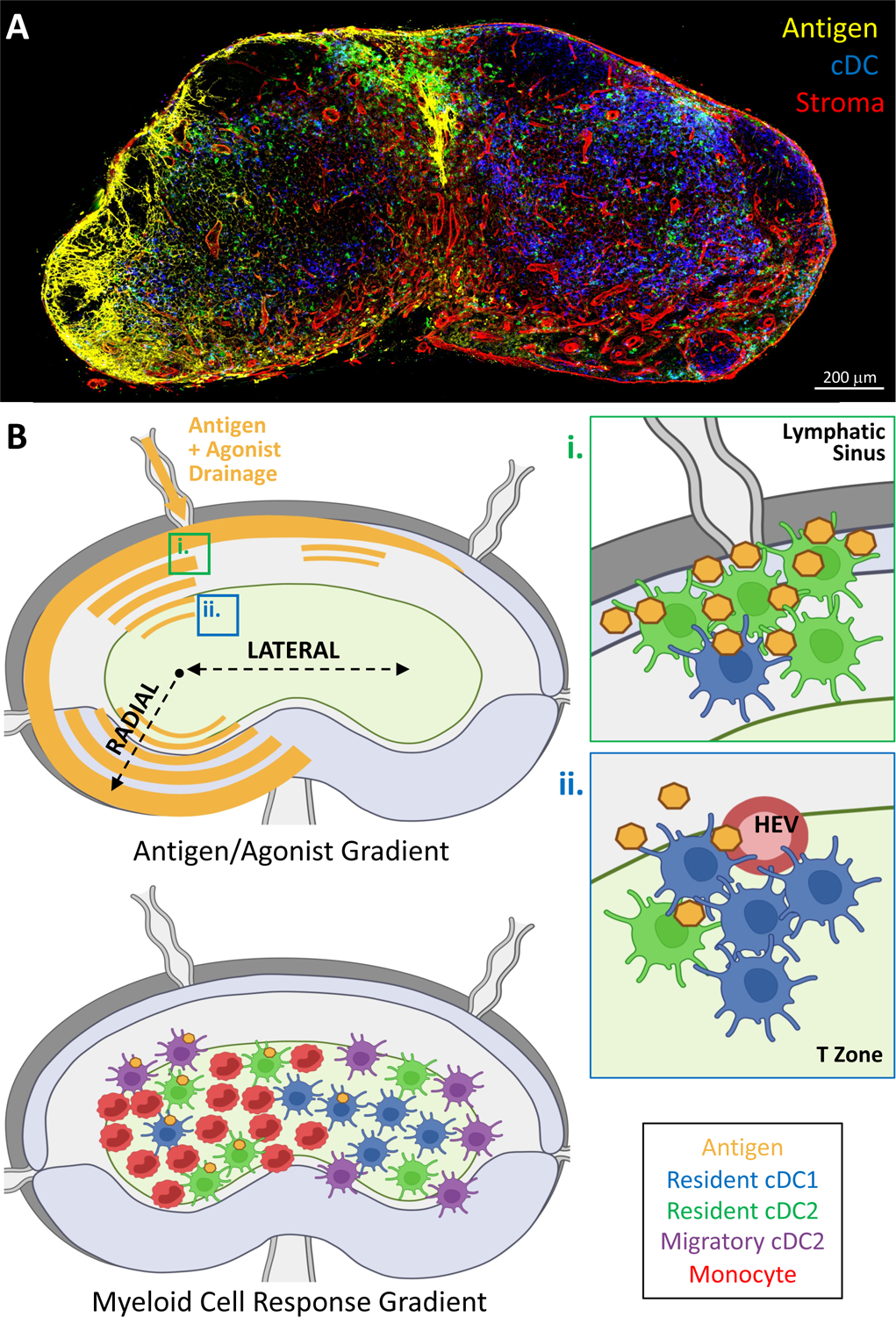
(A) Confocal microscopy image of a draining LN demonstrating gradient formation 2 hours after subcutaneous injection of EαGFP (antigen, yellow) fluorescent protein. cDCs and stromal networks are also revealed with CD11c and Collagen-IV staining, respectively. (B) Schematic showing the radial and lateral gradients of antigen and agonist which are generated within the draining LNs (top). Influence of radial gradients is depicted in the zoom insets (i & ii). (i) Resident cDC2s are enriched near the lymphatic sinuses, and robustly acquire draining antigens. (ii) Lower abundance of antigen is observed within the T cell zone, therefore limiting antigen capture by the more centrally localized cDC1s. Bottom panel depicts the influence of lateral gradients on downstream myeloid cell localization and function.
Created with BioRenderer.com
We have recently demonstrated that in addition to the radial axis of antigen dispersal across the lymphatic and T cell zone regions, lateral dispersal gradients also likely contribute to the generation of immune responses (Figure 3). LNs contain multiple incoming afferent lymphatic vessels, each draining a unique peripheral tissue, which are distributed in distinct sites across the subcapsular sinus. Accordingly, during infection or immunization of a given tissue, the draining node receives incoming stimuli via one dominant lymphatic vessel. This in turn results in markedly greater deposition of antigens and agonists in regions most proximal to the involved afferent vessel, and leads to establishment of lateral gradients across the LN13,28. Concentration of antigen and inflammatory signals on one side of the LN is likely to influence the activation, localization, and function of multiple innate cell types. In this regard, we found highly polarized infiltration of inflammatory monocytes during Type-I inflammation, leading to varied abundance of innate cell subsets across the tissue28. Similar considerations likely apply to migratory cDCs. As various migratory cDC subsets accumulate within the draining LN31,43,207,208, they similarly enter from the specific afferent lymphatic vessel draining the inflamed tissue, and this likely reinforces the polarized distribution of antigen-bearing cDCs across the LN parenchyma. Consistent with this idea, in a variety of infection settings, more robust migratory cDC-driven T cell priming can be observed on one side of the draining LN31,107,209,210. Together, this lateral axis of innate cell composition and activity sets up the generation of multiple distinct microenvironments within a single LN, in which T cells experience divergent conditions during early activation dependent on their localization28. In turn, this imparts distinct differentiation properties to the responding T cells. As we demonstrated in settings of Type-I inflammation, increased infiltration of IL-12-producing activated monocytes into one side of the LN after immunization results in the localized generation of highly differentiated effector T cells. Conversely, less differentiated T cells expressing markers of memory precursor cells are preferentially observed in regions with fewer inflammatory monocytes and a greater abundance of cDCs28. We thus propose that the fundamental rules of lymphatic flow and information dispersal across the LN can directly regulate the heterogeneity of the resultant T cell response.
CONCLUSIONS
Overall, these studies reveal several key features of how immunity is generated and provide important considerations for rational vaccine design. In particular, our past observations of reduced antigen capture by resident cDC1s and less efficient CD8 T cell activation during subunit immunization may have critical implications for vaccine outcomes. In line with this, while subunit vaccines have been shown to generate robust CD4 T cell and antibody responses in humans, they do not appear to induce robust CD8 T cell responses, even when delivered with potent adjuvants211,212. Thus, strategies to better retain antigens within the draining LN, while also promoting higher diffusive properties, may promote more potent CD8 T cell responses during vaccination168,169. In this regard, vaccine formulations that are designed to promote long-term antigen persistence in draining LNs have been shown to augment CD8 T cell responses213–215. Work by many groups, including ours, also highlight the notion that functional cooperation between diverse innate cell types is intimately involved in regulating the magnitude and quality of both cell-mediated and humoral immunity. Therefore, vaccine strategies that simultaneously target multiple innate cell populations should be explored. Finally, it should be noted that different routes of immunization can induce distinct kinetics and biodistribution of vaccine derived materials. This can influence myeloid cell maturation and function as well as downstream adaptive immunity, which can in turn further shape vaccine outcomes216,217.
In sum, the lymphatics are an information-rich superhighway that enables efficient crosstalk between peripheral tissues and the draining LNs. Such transmission of information is necessary for promoting immune quiescence at steady state, as well as for mounting productive immune responses during inflammation. During microbial infection or vaccination, the spatial organization of innate cells within LNs permits effective sampling of information and threat detection, as well as facilitates highly coordinated crosstalk among different immune cells to drive the generation of functional adaptive responses. During Type-II inflammation of barrier surfaces, information on local tissue damage is potently integrated by peripheral tissue cDCs, and is delivered into draining LNs to promote alternate forms of T cell activation geared to this type of insult. At the same time, the drainage patterns of antigens and agonists, coupled with localized innate cell positioning, generates discrete immune microenvironments which control the magnitude, quality, and heterogeneity of downstream adaptive immunity. Together, these large-scale and spatially orchestrated crosstalk events across tissues and among immune cell populations represent the mechanisms responsible for the appropriate generation of immune responses to distinct classes of stimuli.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health awards R01AI134713, R21AI142667, T32GM007270 (J.H.), F31AI161316 (J.H.), and the National Science Foundation Graduate Research Fellowship (NSF DGE-1762114; M.LC.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell. Jul 23 2020;182(2):270–296. doi: 10.1016/j.cell.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. Jul 10 2020;369(6500)doi: 10.1126/science.aax4063 [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The Lymphatic System: Integral Roles in Immunity. Annu Rev Immunol. Apr 26 2017;35:31–52. doi: 10.1146/annurev-immunol-041015-055354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement CC, Cannizzo ES, Nastke MD, et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One. Mar 26 2010;5(3):e9863. doi: 10.1371/journal.pone.0009863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. Dec 21 2009;206(13):2925–35. doi: 10.1084/jem.20091739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schineis P, Runge P, Halin C. Cellular traffic through afferent lymphatic vessels. Vascul Pharmacol. Jan 2019;112:31–41. doi: 10.1016/j.vph.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 7.Stern LJ, Santambrogio L. The melting pot of the MHC II peptidome. Curr Opin Immunol. Jun 2016;40:70–7. doi: 10.1016/j.coi.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. Oct 1 2007;204(10):2349–62. doi: 10.1084/jem.20062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bovay E, Sabine A, Prat-Luri B, et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med. Nov 5 2018;215(11):2760–2777. doi: 10.1084/jem.20180217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogoslowski A, Kubes P. Lymph Nodes: The Unrecognized Barrier against Pathogens. ACS Infect Dis. Aug 10 2018;4(8):1158–1161. doi: 10.1021/acsinfecdis.8b00111 [DOI] [PubMed] [Google Scholar]
- 11.Adair TH, Moffatt DS, Paulsen AW, Guyton AC. Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol. Sep 1982;243(3):H351–9. doi: 10.1152/ajpheart.1982.243.3.H351 [DOI] [PubMed] [Google Scholar]
- 12.Czepielewski RS, Randolph GJ. Lymph nodes go with the flow. J Exp Med. Nov 5 2018;215(11):2699–2701. doi: 10.1084/jem.20181898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med. Oct 2 2017;214(10):3105–3122. doi: 10.1084/jem.20170335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kähäri L, Fair-Mäkelä R, Auvinen K, et al. Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J Clin Invest. Jun 24 2019;129(8):3086–3102. doi: 10.1172/jci125740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi H, Kastenmüller W, Germain RN. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol. 2014;30:141–67. doi: 10.1146/annurev-cellbio-100913-013254 [DOI] [PubMed] [Google Scholar]
- 16.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. Feb 2019;19(2):89–103. doi: 10.1038/s41577-018-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant SM, Lou M, Yao L, Germain RN, Radtke AJ. The lymph node at a glance - how spatial organization optimizes the immune response. J Cell Sci. Mar 6 2020;133(5)doi: 10.1242/jcs.241828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4(5–6):424–36. doi: 10.1159/000337007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran I, Grootveld AK, Nguyen A, Phan TG. Subcapsular Sinus Macrophages: The Seat of Innate and Adaptive Memory in Murine Lymph Nodes. Trends Immunol. Jan 2019;40(1):35–48. doi: 10.1016/j.it.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 20.Gray EE, Friend S, Suzuki K, Phan TG, Cyster JG. Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes. PLoS One. 2012;7(6):e38258. doi: 10.1371/journal.pone.0038258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. Sep 14 2012;150(6):1235–48. doi: 10.1016/j.cell.2012.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Roth TL, Gray EE, et al. Migratory and adhesive cues controlling innate-like lymphocyte surveillance of the pathogen-exposed surface of the lymph node. Elife. Aug 3 2016;5 doi: 10.7554/eLife.18156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chtanova T, Han SJ, Schaeffer M, et al. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. Aug 21 2009;31(2):342–55. doi: 10.1016/j.immuni.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. Mar 21 2013;38(3):502–13. doi: 10.1016/j.immuni.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Böttcher JP, Beyer M, Meissner F, et al. Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nat Commun. Sep 25 2015;6:8306. doi: 10.1038/ncomms9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. Aug 24 2012;37(2):364–76. doi: 10.1016/j.immuni.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. Jan 20 2015;42(1):172–85. doi: 10.1016/j.immuni.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 28.Leal JM, Huang JY, Kohli K, et al. Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci Immunol. Feb 12 2021;6(56)doi: 10.1126/sciimmunol.abb9435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoltzfus CR, Filipek J, Gern BH, et al. CytoMAP: A Spatial Analysis Toolbox Reveals Features of Myeloid Cell Organization in Lymphoid Tissues. Cell Rep. Apr 21 2020;31(3):107523. doi: 10.1016/j.celrep.2020.107523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eickhoff S, Brewitz A, Gerner MY, et al. Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell. Sep 10 2015;162(6):1322–37. doi: 10.1016/j.cell.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity. Sep 15 2015;43(3):554–65. doi: 10.1016/j.immuni.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 32.Ruhland MK, Roberts EW, Cai E, et al. Visualizing Synaptic Transfer of Tumor Antigens among Dendritic Cells. Cancer Cell. Jun 8 2020;37(6):786–799.e5. doi: 10.1016/j.ccell.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. Nov 13 2011;479(7374):542–6. doi: 10.1038/nature10540 [DOI] [PubMed] [Google Scholar]
- 34.Granot T, Senda T, Carpenter DJ, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. March 21 2017;46(3):504–515. doi: 10.1016/j.immuni.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baratin M, Foray C, Demaria O, et al. Homeostatic NF-κB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity. Apr 21 2015;42(4):627–39. doi: 10.1016/j.immuni.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis ESC. Dendritic Cells Revisited. Annu Rev Immunol. Apr 26 2021;39:131–166. doi: 10.1146/annurev-immunol-061020-053707 [DOI] [PubMed] [Google Scholar]
- 37.Devi KS, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. Feb 2017;39(2):137–152. doi: 10.1007/s00281-016-0602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. May 2011;241(1):206–27. doi: 10.1111/j.1600-065X.2011.01015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastow CR, Bunting MD, Kara EE, et al. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc Natl Acad Sci U S A. Apr 27 2021;118(17)doi: 10.1073/pnas.2025763118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber M, Hauschild R, Schwarz J, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. Jan 18 2013;339(6117):328–32. doi: 10.1126/science.1228456 [DOI] [PubMed] [Google Scholar]
- 41.Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. January 2017;17(1):30–48. doi: 10.1038/nri.2016.116 [DOI] [PubMed] [Google Scholar]
- 42.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. May 2005;22(5):643–54. doi: 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 43.Kitano M, Yamazaki C, Takumi A, et al. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A. Jan 26 2016;113(4):1044–9. doi: 10.1073/pnas.1513607113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connor LM, Tang SC, Cognard E, et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J Exp Med. Jan 2017;214(1):125–142. doi: 10.1084/jem.20160470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b⁺ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. Oct 17 2013;39(4):733–43. doi: 10.1016/j.immuni.2013.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochiai S, Roediger B, Abtin A, et al. CD326(lo)CD103(lo)CD11b(lo) dermal dendritic cells are activated by thymic stromal lymphopoietin during contact sensitization in mice. J Immunol. Sep 1 2014;193(5):2504–11. doi: 10.4049/jimmunol.1400536 [DOI] [PubMed] [Google Scholar]
- 47.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol. Jun 2014;14(6):377–91. doi: 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. Dec 10 2015;528(7581):225–30. doi: 10.1038/nature16169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong HS, Park K, Gola A, et al. A local regulatory T cell feedback circuit maintains immune homeostasis by pruning self-activated T cells. Cell. Jul 22 2021;184(15):3981–3997.e22. doi: 10.1016/j.cell.2021.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smigiel KS, Richards E, Srivastava S, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. Jan 13 2014;211(1):121–36. doi: 10.1084/jem.20131142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buszko M, Shevach EM. Control of regulatory T cell homeostasis. Curr Opin Immunol. Dec 2020;67:18–26. doi: 10.1016/j.coi.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 53.Alexandre YO, Mueller SN. Stromal cell networks coordinate immune response generation and maintenance. Immunol Rev. May 2018;283(1):77–85. doi: 10.1111/imr.12641 [DOI] [PubMed] [Google Scholar]
- 54.Rodda LB, Lu E, Bennett ML, et al. Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity. May 15 2018;48(5):1014–1028.e6. doi: 10.1016/j.immuni.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnamurty AT, Turley SJ. Lymph node stromal cells: cartographers of the immune system. Nat Immunol. Apr 2020;21(4):369–380. doi: 10.1038/s41590-020-0635-3 [DOI] [PubMed] [Google Scholar]
- 56.Pikor NB, Cheng HW, Onder L, Ludewig B. Development and Immunological Function of Lymph Node Stromal Cells. J Immunol. January 15 2021;206(2):257–263. doi: 10.4049/jimmunol.2000914 [DOI] [PubMed] [Google Scholar]
- 57.Kapoor VN, Müller S, Keerthivasan S, et al. Gremlin 1(+) fibroblastic niche maintains dendritic cell homeostasis in lymphoid tissues. Nat Immunol. May 2021;22(5):571–585. doi: 10.1038/s41590-021-00920-6 [DOI] [PubMed] [Google Scholar]
- 58.Pikor NB, Mörbe U, Lütge M, et al. Remodeling of light and dark zone follicular dendritic cells governs germinal center responses. Nat Immunol. June 2020;21(6):649–659. doi: 10.1038/s41590-020-0672-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denton AE, Carr EJ, Magiera LP, Watts AJB, Fearon DT. Embryonic FAP. J Exp Med. October 07 2019;216(10):2242–2252. doi: 10.1084/jem.20181705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camara A, Cordeiro OG, Alloush F, et al. Lymph Node Mesenchymal and Endothelial Stromal Cells Cooperate via the RANK-RANKL Cytokine Axis to Shape the Sinusoidal Macrophage Niche. Immunity. Jun 18 2019;50(6):1467–1481.e6. doi: 10.1016/j.immuni.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 61.Mondor I, Baratin M, Lagueyrie M, et al. Lymphatic Endothelial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity. Jun 18 2019;50(6):1453–1466.e4. doi: 10.1016/j.immuni.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulvmar MH, Werth K, Braun A, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol. Jul 2014;15(7):623–30. doi: 10.1038/ni.2889 [DOI] [PubMed] [Google Scholar]
- 63.Bajénoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. Dec 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crotty S T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. May 21 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.León B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. May 27 2012;13(7):681–90. doi: 10.1038/ni.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Cho B, Suzuki K, et al. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med. Nov 21 2011;208(12):2497–510. doi: 10.1084/jem.20111449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. Nov 2007;8(11):1255–65. doi: 10.1038/ni1513 [DOI] [PubMed] [Google Scholar]
- 68.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. Nov 7 2000;97(23):12694–9. doi: 10.1073/pnas.97.23.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. Sep 2009;9(9):618–29. doi: 10.1038/nri2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schumann K, Lämmermann T, Bruckner M, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. May 28 2010;32(5):703–13. doi: 10.1016/j.immuni.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 71.Gatto D, Wood K, Caminschi I, et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol. May 2013;14(5):446–53. doi: 10.1038/ni.2555 [DOI] [PubMed] [Google Scholar]
- 72.Lu E, Dang EV, McDonald JG, Cyster JG. Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci Immunol. Apr 7 2017;2(10)doi: 10.1126/sciimmunol.aal5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi T, Cyster JG. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. Elife. May 14 2013;2:e00757. doi: 10.7554/eLife.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baptista AP, Gola A, Huang Y, et al. The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4(+) T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity. May 21 2019;50(5):1188–1201.e6. doi: 10.1016/j.immuni.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 75.Brulois K, Rajaraman A, Szade A, et al. A molecular map of murine lymph node blood vascular endothelium at single cell resolution. Nat Commun. Jul 30 2020;11(1):3798. doi: 10.1038/s41467-020-17291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veerman K, Tardiveau C, Martins F, Coudert J, Girard JP. Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes. Cell Rep. Mar 12 2019;26(11):3116–3131.e5. doi: 10.1016/j.celrep.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 77.Cabeza-Cabrerizo M, van Blijswijk J, Wienert S, et al. Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci Immunol. Mar 1 2019;4(33)doi: 10.1126/sciimmunol.aaw1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grajales-Reyes GE, Iwata A, Albring J, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α(+) conventional DC clonogenic progenitor. Nat Immunol. Jul 2015;16(7):708–17. doi: 10.1038/ni.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schlitzer A, Sivakamasundari V, Chen J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. Jul 2015;16(7):718–28. doi: 10.1038/ni.3200 [DOI] [PubMed] [Google Scholar]
- 80.Baptista AP, Gerner MY. Lymphoid stromal cells proGrem dendritic cell homeostasis. Nat Immunol. May 2021;22(5):541–543. doi: 10.1038/s41590-021-00924-2 [DOI] [PubMed] [Google Scholar]
- 81.Acton SE, Astarita JL, Malhotra D, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. Aug 24 2012;37(2):276–89. doi: 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acton SE, Farrugia AJ, Astarita JL, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. Oct 23 2014;514(7523):498–502. doi: 10.1038/nature13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Astarita JL, Cremasco V, Fu J, et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. Jan 2015;16(1):75–84. doi: 10.1038/ni.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dasoveanu DC, Shipman WD, Chia JJ, Chyou S, Lu TT. Regulation of Lymph Node Vascular-Stromal Compartment by Dendritic Cells. Trends Immunol. Nov 2016;37(11):764–777. doi: 10.1016/j.it.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar V, Dasoveanu DC, Chyou S, et al. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity. Apr 21 2015;42(4):719–30. doi: 10.1016/j.immuni.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. Jul 2007;27(1):160–71. doi: 10.1016/j.immuni.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 87.Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. Nov 1 2007;450(7166):110–4. doi: 10.1038/nature06287 [DOI] [PubMed] [Google Scholar]
- 88.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. Sep 2007;8(9):992–1000. doi: 10.1038/ni1494 [DOI] [PubMed] [Google Scholar]
- 89.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. Jul 2009;10(7):786–93. doi: 10.1038/ni.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farrell HE, Bruce K, Lawler C, Cardin RD, Davis-Poynter NJ, Stevenson PG. Type 1 Interferons and NK Cells Limit Murine Cytomegalovirus Escape from the Lymph Node Subcapsular Sinus. PLoS Pathog. Dec 2016;12(12):e1006069. doi: 10.1371/journal.ppat.1006069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iannacone M, Moseman EA, Tonti E, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. Jun 24 2010;465(7301):1079–83. doi: 10.1038/nature09118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moseman EA, Iannacone M, Bosurgi L, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. Mar 23 2012;36(3):415–26. doi: 10.1016/j.immuni.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sagoo P, Garcia Z, Breart B, et al. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat Med. Jan 2016;22(1):64–71. doi: 10.1038/nm.4016 [DOI] [PubMed] [Google Scholar]
- 94.Bogoslowski A, Butcher EC, Kubes P. Neutrophils recruited through high endothelial venules of the lymph nodes via PNAd intercept disseminating Staphylococcus aureus. Proc Natl Acad Sci U S A. Mar 6 2018;115(10):2449–2454. doi: 10.1073/pnas.1715756115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lämmermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. Jun 20 2013;498(7454):371–5. doi: 10.1038/nature12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kienle K, Glaser KM, Eickhoff S, et al. Neutrophils self-limit swarming to contain bacterial growth in vivo. Science. Jun 18 2021;372(6548)doi: 10.1126/science.abe7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sung JH, Zhang H, Moseman EA, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. Sep 14 2012;150(6):1249–63. doi: 10.1016/j.cell.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guarda G, Hons M, Soriano SF, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. Jul 2007;8(7):743–52. doi: 10.1038/ni1469 [DOI] [PubMed] [Google Scholar]
- 99.Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. Jul 2003;19(1):47–57. doi: 10.1016/s1074-7613(03)00175-4 [DOI] [PubMed] [Google Scholar]
- 100.Woodruff MC, Heesters BA, Herndon CN, et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J Exp Med. Jul 28 2014;211(8):1611–21. doi: 10.1084/jem.20132327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calabro S, Liu D, Gallman A, et al. Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep. August 30 2016;16(9):2472–85. doi: 10.1016/j.celrep.2016.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Koker S, Van Hoecke L, De Beuckelaer A, et al. Inflammatory monocytes regulate Th1 oriented immunity to CpG adjuvanted protein vaccines through production of IL-12. Sci Rep. Jul 20 2017;7(1):5986. doi: 10.1038/s41598-017-06236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lian J, Ozga AJ, Sokol CL, Luster AD. Targeting Lymph Node Niches Enhances Type 1 Immune Responses to Immunization. Cell Rep. May 26 2020;31(8):107679. doi: 10.1016/j.celrep.2020.107679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakano H, Lin KL, Yanagita M, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. Apr 2009;10(4):394–402. doi: 10.1038/ni.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Groom JR. Regulators of T-cell fate: Integration of cell migration, differentiation and function. Immunol Rev. May 2019;289(1):101–114. doi: 10.1111/imr.12742 [DOI] [PubMed] [Google Scholar]
- 106.Blecher-Gonen R, Bost P, Hilligan KL, et al. Single-Cell Analysis of Diverse Pathogen Responses Defines a Molecular Roadmap for Generating Antigen-Specific Immunity. Cell Syst. Feb 27 2019;8(2):109–121.e6. doi: 10.1016/j.cels.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 107.Duckworth BC, Lafouresse F, Wimmer VC, et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat Immunol. Apr 2021;22(4):434–448. doi: 10.1038/s41590-021-00878-5 [DOI] [PubMed] [Google Scholar]
- 108.Groom JR, Richmond J, Murooka TT, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. Dec 14 2012;37(6):1091–103. doi: 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hilligan KL, Tang SC, Hyde EJ, et al. Dermal IRF4+ dendritic cells and monocytes license CD4+ T helper cells to distinct cytokine profiles. Nat Commun. Nov 6 2020;11(1):5637. doi: 10.1038/s41467-020-19463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mendoza A, Yewdell WT, Hoyos B, et al. Assembly of a spatial circuit of T-bet-expressing T and B lymphocytes is required for antiviral humoral immunity. Sci Immunol. Jun 11 2021;6(60)doi: 10.1126/sciimmunol.abi4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexandre YO, Ghilas S, Sanchez C, Le Bon A, Crozat K, Dalod M. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J Exp Med. Jan 11 2016;213(1):75–92. doi: 10.1084/jem.20142350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Askenase MH, Han SJ, Byrd AL, et al. Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity. Jun 16 2015;42(6):1130–42. doi: 10.1016/j.immuni.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldszmid RS, Caspar P, Rivollier A, et al. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. Jun 29 2012;36(6):1047–59. doi: 10.1016/j.immuni.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghilas S, Ambrosini M, Cancel JC, et al. Natural killer cells and dendritic epidermal γδ T cells orchestrate type 1 conventional DC spatiotemporal repositioning toward CD8(+) T cells. iScience. Sep 24 2021;24(9):103059. doi: 10.1016/j.isci.2021.103059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee AJ, Chen B, Chew MV, et al. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J Exp Med. Apr 3 2017;214(4):1153–1167. doi: 10.1084/jem.20160880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brewitz A, Eickhoff S, Dähling S, et al. CD8(+) T Cells Orchestrate pDC-XCR1(+) Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity. Feb 21 2017;46(2):205–219. doi: 10.1016/j.immuni.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Askew D, Su CA, Barkauskas DS, et al. Transient Surface CCR5 Expression by Naive CD8+ T Cells within Inflamed Lymph Nodes Is Dependent on High Endothelial Venule Interaction and Augments Th Cell-Dependent Memory Response. J Immunol. May 1 2016;196(9):3653–64. doi: 10.4049/jimmunol.1501176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. Apr 13 2006;440(7086):890–5. doi: 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- 119.Hickman HD, Li L, Reynoso GV, et al. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med. Nov 21 2011;208(12):2511–24. doi: 10.1084/jem.20102545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hugues S, Scholer A, Boissonnas A, et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. Sep 2007;8(9):921–30. doi: 10.1038/ni1495 [DOI] [PubMed] [Google Scholar]
- 121.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. Jan 15 2007;178(2):778–87. doi: 10.4049/jimmunol.178.2.778 [DOI] [PubMed] [Google Scholar]
- 122.Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. Jun 2020;17(6):587–599. doi: 10.1038/s41423-020-0465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin C, Bonduelle O, Nuttens C, et al. Critical Role for Skin-Derived Migratory DCs and Langerhans Cells in T. J Invest Dermatol. September 2017;137(9):1905–1913. doi: 10.1016/j.jid.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 124.Igyártó BZ, Haley K, Ortner D, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. Aug 26 2011;35(2):260–72. doi: 10.1016/j.immuni.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee HK, Zamora M, Linehan MM, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. Feb 16 2009;206(2):359–70. doi: 10.1084/jem.20080601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martínez-López M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol. Jan 2015;45(1):119–29. doi: 10.1002/eji.201444651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. Aug 1 2006;177(3):1618–27. doi: 10.4049/jimmunol.177.3.1618 [DOI] [PubMed] [Google Scholar]
- 128.Bosteels C, Neyt K, Vanheerswynghels M, et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity. Jun 16 2020;52(6):1039–1056.e9. doi: 10.1016/j.immuni.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol. April 26 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215 [DOI] [PubMed] [Google Scholar]
- 130.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. Jul 2006;25(1):153–62. doi: 10.1016/j.immuni.2006.04.017 [DOI] [PubMed] [Google Scholar]
- 131.Kedl RM, Lindsay RS, Finlon JM, Lucas ED, Friedman RS, Tamburini BAJ. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Commun. Dec 11 2017;8(1):2034. doi: 10.1038/s41467-017-02247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. Jun 6 2014;5:3989. doi: 10.1038/ncomms4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walsh SM, Sheridan RM, Lucas ED, et al. Molecular tracking devices quantify antigen distribution and archiving in the murine lymph node. Elife. Apr 12 2021;10doi: 10.7554/eLife.62781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu J, Jankovic D, Oler AJ, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. Oct 19 2012;37(4):660–73. doi: 10.1016/j.immuni.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Everts B, Tussiwand R, Dreesen L, et al. Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12. J Exp Med. Jan 11 2016;213(1):35–51. doi: 10.1084/jem.20150235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conejero L, Khouili SC, Martínez-Cano S, Izquierdo HM, Brandi P, Sancho D. Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. JCI Insight. May 18 2017;2(10)doi: 10.1172/jci.insight.90420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao Y, Nish SA, Jiang R, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. Oct 17 2013;39(4):722–32. doi: 10.1016/j.immuni.2013.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williams JW, Tjota MY, Clay BS, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. Feb 21 2013;38(2):322–35. doi: 10.1016/j.immuni.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 140.Tussiwand R, Everts B, Grajales-Reyes GE, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. May 19 2015;42(5):916–28. doi: 10.1016/j.immuni.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin X, Chen S, Eisenbarth SC. Dendritic Cell Regulation of T Helper Cells. Annu Rev Immunol. Apr 26 2021;39:759–790. doi: 10.1146/annurev-immunol-101819-025146 [DOI] [PubMed] [Google Scholar]
- 142.Sokol CL, Camire RB, Jones MC, Luster AD. The Chemokine Receptor CCR8 Promotes the Migration of Dendritic Cells into the Lymph Node Parenchyma to Initiate the Allergic Immune Response. Immunity. September 18 2018;49(3):449–463.e6. doi: 10.1016/j.immuni.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Q, Liu Z, Rozo CT, et al. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. Sep 15 2007;179(6):3821–30. doi: 10.4049/jimmunol.179.6.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. Dec 10 1999;286(5447):2159–62. doi: 10.1126/science.286.5447.2159 [DOI] [PubMed] [Google Scholar]
- 145.León B, Lund FE. Compartmentalization of dendritic cell and T-cell interactions in the lymph node: Anatomy of T-cell fate decisions. Immunol Rev. May 2019;289(1):84–100. doi: 10.1111/imr.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. Nov 07 2005;202(9):1213–23. doi: 10.1084/jem.20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kitajima M, Ziegler SF. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol. Nov 15 2013;191(10):4903–7. doi: 10.4049/jimmunol.1302175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tindemans I, Peeters MJW, Hendriks RW. Notch Signaling in T Helper Cell Subsets: Instructor or Unbiased Amplifier? Front Immunol. 2017;8:419. doi: 10.3389/fimmu.2017.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walker JA, McKenzie ANJ. T H 2 cell development and function. Nat Rev Immunol. February 2018;18(2):121–133. doi: 10.1038/nri.2017.118 [DOI] [PubMed] [Google Scholar]
- 150.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. May 11 2009;206(5):1001–7. doi: 10.1084/jem.20090313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. May 11 2009;206(5):991–9. doi: 10.1084/jem.20090303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Prout MS, Kyle RL, Ronchese F, Le Gros G. IL-4 Is a Key Requirement for IL-4- and IL-4/IL-13-Expressing CD4 Th2 Subsets in Lung and Skin. Front Immunol. 2018;9:1211. doi: 10.3389/fimmu.2018.01211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. Mar 2008;9(3):310–8. doi: 10.1038/ni1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tang H, Cao W, Kasturi SP, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. Jul 2010;11(7):608–17. doi: 10.1038/ni.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. Jul 2009;10(7):713–20. doi: 10.1038/ni.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. Sep 27 2010;207(10):2097–111. doi: 10.1084/jem.20101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. Feb 01 2010;184(3):1143–7. doi: 10.4049/jimmunol.0902447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He K, Hettinga A, Kale SL, et al. Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J Exp Med. July 06 2020;217(7)doi: 10.1084/jem.20190742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. Apr 2010;10(4):225–35. doi: 10.1038/nri2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. Jul 17 2014;41(1):63–74. doi: 10.1016/j.immuni.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Panhuys N TCR Signal Strength Alters T-DC Activation and Interaction Times and Directs the Outcome of Differentiation. Front Immunol. 2016;7:6. doi: 10.3389/fimmu.2016.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. May 05 2016;533(7601):110–4. doi: 10.1038/nature17947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnaswamy JK, Gowthaman U, Zhang B, et al. Migratory CD11b + conventional dendritic cells induce T follicular helper cell–dependent antibody responses. Sci Immunol. December 01 2017;2(18)doi: 10.1126/sciimmunol.aam9169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.De Giovanni M, Cutillo V, Giladi A, et al. Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4. Nat Immunol. March 2020;21(3):321–330. doi: 10.1038/s41590-020-0596-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumamoto Y, Hirai T, Wong PW, Kaplan DH, Iwasaki A. CD301b + dendritic cells suppress T follicular helper cells and antibody responses to protein antigens. Elife. Sep 22 2016;5doi: 10.7554/eLife.17979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. Jul 06 2009;206(7):1589–602. doi: 10.1084/jem.20090247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. Mar 15 2010;184(6):2751–5. doi: 10.4049/jimmunol.0903201 [DOI] [PubMed] [Google Scholar]
- 168.Irvine DJ, Aung A, Silva M. Controlling timing and location in vaccines. Adv Drug Deliv Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater. Jun 2019;4(6):415–428. doi: 10.1038/s41578-019-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. Jul 23 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cayrol C, Duval A, Schmitt P, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. April 2018;19(4):375–385. doi: 10.1038/s41590-018-0067-5 [DOI] [PubMed] [Google Scholar]
- 172.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. Jul 27 2012;337(6093):431–5. doi: 10.1126/science.1221064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wan H, Winton HL, Soeller C, et al. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. May 2000;30(5):685–98. doi: 10.1046/j.1365-2222.2000.00820.x [DOI] [PubMed] [Google Scholar]
- 174.Sharafutdinov I, Esmaeili DS, Harrer A, Tegtmeyer N, Sticht H, Backert S. Serine Protease HtrA Cleaves the Tight Junction Component Claudin-8. Front Cell Infect Microbiol. 2020;10:590186. doi: 10.3389/fcimb.2020.590186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen- induced inflammatory responses. Chem Immunol Allergy. 2006;90:45–64. doi: 10.1159/000088880 [DOI] [PubMed] [Google Scholar]
- 176.Caffrey CR, Goupil L, Rebello KM, Dalton JP, Smith D. Cysteine proteases as digestive enzymes in parasitic helminths. PLoS Negl Trop Dis. August 2018;12(8):e0005840. doi: 10.1371/journal.pntd.0005840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schneider C, Shen C, Gopal AA, et al. Migration-induced cell shattering due to DOCK8 deficiency causes a type 2-biased helper T cell response. Nat Immunol. December 2020;21(12):1528–1539. doi: 10.1038/s41590-020-0795-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ronchese F, Hilligan KL, Mayer JU. Dendritic cells and the skin environment. Curr Opin Immunol. May 5 2020;64:56–62. doi: 10.1016/j.coi.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 179.Sumpter TL, Balmert SC, Kaplan DH. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight. Jan 10 2019;4(1)doi: 10.1172/jci.insight.123947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. April 01 2019;129(4):1441–1451. doi: 10.1172/JCI124606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bell BD, Kitajima M, Larson RP, et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. Apr 2013;14(4):364–71. doi: 10.1038/ni.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Larson RP, Zimmerli SC, Comeau MR, et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J Immunol. Mar 15 2010;184(6):2974–84. doi: 10.4049/jimmunol.0803478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. May 2009;123(5):1047–54. doi: 10.1016/j.jaci.2009.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. Jun 2011;41(6):1675–86. doi: 10.1002/eji.201041033 [DOI] [PubMed] [Google Scholar]
- 185.Chu DK, Llop-Guevara A, Walker TD, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. Jan 2013;131(1):187–200.e1–8. doi: 10.1016/j.jaci.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 186.de Kleer IM, Kool M, de Bruijn MJ, et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity. December 20 2016;45(6):1285–1298. doi: 10.1016/j.immuni.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 187.Carroll-Portillo A, Cannon JL, te Riet J, et al. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J Cell Biol. Aug 31 2015;210(5):851–64. doi: 10.1083/jcb.201412074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shimokawa C, Kanaya T, Hachisuka M, et al. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity. May 16 2017;46(5):863–874.e4. doi: 10.1016/j.immuni.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 189.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. Apr 01 2006;176(7):4102–12. doi: 10.4049/jimmunol.176.7.4102 [DOI] [PubMed] [Google Scholar]
- 190.Halim TY, Steer CA, Mathä L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. Mar 20 2014;40(3):425–35. doi: 10.1016/j.immuni.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Halim TY, Hwang YY, Scanlon ST, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. Jan 2016;17(1):57–64. doi: 10.1038/ni.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dahlgren MW, Jones SW, Cautivo KM, et al. Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity. Mar 19 2019;50(3):707–722.e6. doi: 10.1016/j.immuni.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dahlgren MW, Molofsky AB. Adventitial Cuffs: Regional Hubs for Tissue Immunity. Trends Immunol. Oct 2019;40(10):877–887. doi: 10.1016/j.it.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perner C, Flayer CH, Zhu X, et al. Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity. November 17 2020;53(5):1063–1077.e7. doi: 10.1016/j.immuni.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Webb LM, Lundie RJ, Borger JG, et al. Type I interferon is required for T helper (Th) 2 induction by dendritic cells. EMBO J. August 15 2017;36(16):2404–2418. doi: 10.15252/embj.201695345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. Apr 2007;26(4):491–502. doi: 10.1016/j.immuni.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 197.Roozendaal R, Mempel TR, Pitcher LA, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. Feb 20 2009;30(2):264–76. doi: 10.1016/j.immuni.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reynoso GV, Weisberg AS, Shannon JP, et al. Lymph node conduits transport virions for rapid T cell activation. Nat Immunol. May 2019;20(5):602–612. doi: 10.1038/s41590-019-0342-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Acton SE, Onder L, Novkovic M, Martinez VG, Ludewig B. Communication, construction, and fluid control: lymphoid organ fibroblastic reticular cell and conduit networks. Trends Immunol. Sep 2021;42(9):782–794. doi: 10.1016/j.it.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 200.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. Nov 20 2000;192(10):1425–40. doi: 10.1084/jem.192.10.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rantakari P, Auvinen K, Jäppinen N, et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol. Apr 2015;16(4):386–96. doi: 10.1038/ni.3101 [DOI] [PubMed] [Google Scholar]
- 202.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. Jan 2005;22(1):19–29. doi: 10.1016/j.immuni.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 203.Baekkevold ES, Yamanaka T, Palframan RT, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. May 7 2001;193(9):1105–12. doi: 10.1084/jem.193.9.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Palframan RT, Jung S, Cheng G, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. Nov 5 2001;194(9):1361–73. doi: 10.1084/jem.194.9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ohtani O, Ohtani Y, Carati CJ, Gannon BJ. Fluid and cellular pathways of rat lymph nodes in relation to lymphatic labyrinths and Aquaporin-1 expression. Arch Histol Cytol. Aug 2003;66(3):261–72. doi: 10.1679/aohc.66.261 [DOI] [PubMed] [Google Scholar]
- 206.Baptista AP, Gola A, Huang Y, et al. The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4. Immunity. May 2019;50(5):1188–1201.e6. doi: 10.1016/j.immuni.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 207.Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, Aebischer T. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol. Mar 2004;34(3):715–725. doi: 10.1002/eji.200324391 [DOI] [PubMed] [Google Scholar]
- 208.Tomura M, Hata A, Matsuoka S, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep. Aug 12 2014;4:6030. doi: 10.1038/srep06030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, León B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. Feb 16 2016;44(2):259–73. doi: 10.1016/j.immuni.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Perez-Shibayama C, Islander U, Lütge M, et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8(+) T cells. Sci Immunol. Sep 11 2020;5(51)doi: 10.1126/sciimmunol.abb7066 [DOI] [PubMed] [Google Scholar]
- 211.Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb Perspect Med. Sep 2011;1(1):a007252. doi: 10.1101/cshperspect.a007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pennock ND, Kedl JD, Kedl RM. T Cell Vaccinology: Beyond the Reflection of Infectious Responses. Trends Immunol. Mar 2016;37(3):170–180. doi: 10.1016/j.it.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kastenmüller K, Wille-Reece U, Lindsay RW, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest. May 2011;121(5):1782–96. doi: 10.1172/jci45416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lynn GM, Laga R, Darrah PA, et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. Nov 2015;33(11):1201–10. doi: 10.1038/nbt.3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu H, Moynihan KD, Zheng Y, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. Mar 27 2014;507(7493):519–22. doi: 10.1038/nature12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baharom F, Ramirez-Valdez RA, Tobin KKS, et al. Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat Immunol. Jan 2021;22(1):41–52. doi: 10.1038/s41590-020-00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. Jan 2020;577(7788):95–102. doi: 10.1038/s41586-019-1817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


