Abstract
Assessment of asthma comorbidities, conditions that adversely affect the pathobiology of asthma or impair its response to therapies, is a fundamental step in the evaluation and management of patients with difficult-to-treat asthma. Identifying and effectively treating asthma comorbidities, such as obesity, obstructive sleep apnea, and chronic sinusitis with nasal polyps may improve asthma control and reduce exacerbations. Additionally, identifying comorbid T2 inflammatory conditions may help guide optimal selection of biologic therapies. Here, we describe common comorbid conditions found in adult and pediatric difficult-to-control asthma, discuss evidence for the association with asthma morbidity and treatment benefit, and provide information on how and when to assess comorbidities.
Introduction
Asthma comorbidities augment the symptoms of asthma, worsen the intrinsic disease, or provide additional limitations to asthma management. Assessing and treating comorbidities is a fundamental step in evaluating patients with difficult-to-treat asthma. In many cases, appropriate management of the comorbid condition(s) may improve asthma symptoms or morbidity. Additionally, comorbid conditions that share Type 2 inflammatory pathways may inform optimal choice of biologic asthma therapy.
We review the evidence for comorbidities in pediatric and adult patients with difficult-to-control asthma (Table 1), highlighting the domains of asthma control affected, evaluation, and, where available, anticipated asthma outcomes with treatment of the comorbidity (Table 2). We present a clinical pathway for evaluating asthma comorbidities (Figure 2).
Table 1.
Evidence of comorbidity effect on asthma control in adults and children
| Comorbidity | Adult/pediatric | Level of evidence | Affected asthma domain | ||
|---|---|---|---|---|---|
| Patient reported outcomes (Sx/QOL) | Exacerbation | Lung function | |||
| Allergic Rhinitis | Adult | Obs, RCT, Meta | x | x | - |
| Pedi | Meta | x | x | - | |
| CRSsNP | Adult | Obs, RCT | x | x | - |
| Pedi | Obs | - | x | - | |
| CRSwNP | Adult | Obs, RCT | x | x | x |
| Pedi | Obs | - | - | - | |
| Obesity | Adult | Obs, RCT | x | x | x |
| Pedi | Obs, RCT | x | x | x | |
| ILO | Adult | Obs | x | x | - |
| Pedi | Obs | x | x | - | |
| Dysfunctional breathing | Adult | Obs | x | x | |
| Pedi | Obs | x | x | ||
| OSA | Adult | Obs, RCT, Meta | x | x | x |
| Pedi | Obs, RCT, Meta | x | x | - | |
| GERD | Adult | Obs, RCT, Meta | x | x | x |
| Pedi | Obs, RCT, Meta | x | x | - | |
| Anxiety/depression | Adult | Obs | x | x | - |
| Pedi | Obs | x | x | - | |
| Vitamin D deficiency | Adult | Obs, RCT, Meta | x | ||
| Pedi | Obs, RCT, Meta | x | x | x | |
| ABPA/M | Adult | Obs, RCT | x | x | x |
| Pedi | Obs | x | x | ||
| Smoking/SHS | Adult | Obs, RCT | x | x | |
| Pedi | Obs | x | x | x | |
CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; ILO, inducible laryngeal obstruction; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux; ABPA/M. allergic bronchopulmonary aspergillosis/mycosis; SHS (secondhand smoke); Obs, observational; RCT, randomized control trial; Meta, metanalysis; Sx, symptoms; QOL, quality of life; “x” indicates supportive evidence of association with the outcome domain, “-“ indicates studies have not found significant associations, absence of rating indicates a lack of research studies related to the outcome domain.
Table 2.
Clinical evaluation and management of asthma comorbidities
| Comorbidity | Clinical Clues | Suggested Evaluation | Recommended Intervention | Anticipated asthma benefit |
|---|---|---|---|---|
| Allergic Rhinitis | Nasal symptoms | SPT or sIgE | INCS ± oral/nasal antihistamines, montelukast, nasal saline | Uncertain, possible fewer exacerbations |
| CRSwNP | Chronic congestion, sinus pressure, cough | Nasal examination, sinus CT, rhinoscopy; Aspirin sensitivity In children: sweat test, ciliary bx/PCD genetics |
Oral/intranasal steroids, antihistamines, nasal saline, antibiotics, sinus surgery; Aspirin desensitization; anti-IgE, anti-IL-5, IntiIL-4r therapy | Improved symptoms, FEV1, exacerbations |
| Obesity | Elevated BMI | BMI, Metabolic syndrome | Diet, exercise program; bariatric surgery (adult) | Improved QOL, asthma control, FEV1 |
| ILO; | Stridor, discrete episodes, hyperventilation | Laryngoscopy with provocation; | Speech Pathology, stimulus avoidance, inhaled anticholinergics*; psychopharmacologic therapy, if indicated | Improved symptoms |
| Dysfunctional breathing | hyperventilation, sighing, asynchronous thoraco-abdominal breathing | SEBQ/Nijmegen Questionnaire | Breathing retraining | Improved symptoms, QOL |
| OSA | Snoring Daytime somnolence | PSG | Adenotonsillectomy (children) CPAP | Improved exacerbations, symptoms, QOL |
| GERD | Heartburn, regurgitation, chest pain, cough | GI endoscopy, impedence/pH probe | Gastric acid suppression, fundoplication | Slight improved FEV1 and rescue medication use |
| Anxiety/depression | mood/behavioral cues | Screening tools (i.e., GAD7, PHQ9, HADS); psychology referral | CBT, psychopharmacologic therapy | Possible improved symptoms, QOL |
| Vitamin D deficiency | 25 OH Vitamin D level (<30 ng/ml) | Vitamin D supplementation | Possible improved exacerbation rate in adults achieving normal Vitamin D levels | |
| ABPA/M | Uncontrolled asthma, bronchitis, mucus plugs | Skin test/sIgE to fungus, total IgE, aspergillus precipitins or sIgG**; CXR; chest CT | Systemic corticosteroids + antifungal agent; alternative: omalizumab | Symptoms, lung function |
| Smoking/SHS | History, observed odor of smoke | History, urinary cotinine | Smoking cessation counseling, medical management | Symptoms, lung function, exacerbations |
| COPD | Dyspnea, chronic cough, sputum production | History, pre-and post spirometry | Smoking cessation; Asthma pharmacotherapy; LAMA-LABA-ICS therapy | Symptoms, lung function, exacerbations |
CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; ILO, inducible laryngeal obstruction; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux; ABPA/M. allergic bronchopulmonary aspergillosis/mycosis; SHS (secondhand smoke); PSG, polysomnography; sIgE, specific Immunoglobulin E; CT, computed tomography; CPAP, continuous positive airway pressure; CBT, cognitive behavioral therapy; FEV1, forced exhalatory volume in one second; QOL, quality of life; GAD7, General Anxiety Disorder-7; PHQ9, Patient Health Questionnaire-9; HADS, Hospital Anxiety and Depression Scale; ICS, inhaled corticosteroids:*anecdotal evidence; ** ABPA diagnostic criteria: (1) predisposing asthma or CF, (2) Aspergillus skin test reactivity or detectable serum IgE to Aspergillus fumigatus, (3) total serum IgE >1000 IU/ml (lower levels acceptable if patient meets all other criteria), (4) at least two of the following: Precipitating antibodies or increased Aspergillus species IgG level; chest radiographic infiltrates; Total eosinophil count >500 cells/microL in glucocorticoid-naïve patients (may be historical).
Comorbidities
Allergic Rhinitis
Epidemiological evidence supports the coexistence of asthma and upper airway disorders, including allergic rhinitis and chronic rhinosinusitis (CRS) with or without nasal polyposis1–4. The upper and lower airways are linked by shared exposure to air pollutants and aeroallergens and respond via similar pathophysiologic mechanisms5.
Allergic rhinitis is characterized as nasal congestion, rhinorrhea, and sneezing, often associated with itchiness of the eyes, nose and palate, post-nasal drip, throat clearing, and cough, in response to an allergen exposure in a sensitized individual. The diagnosis is typically made on clinical grounds based on presence of these symptoms, evidence on nasal mucosal edema, rhinorrhea, and facial features such as infraorbital edema and darkening (“allergic shiners”), Dennie-Morgan lines, and transverse nasal crease. Many patients are treated empirically, but allergen diagnostic testing by skin prick with allergen extracts or specific IgE serum antibodies, can differentiate allergic from non-allergic rhinitis and lead to allergen-specific therapies, such as environmental controls or allergen immunotherapy.
Asthma and allergic rhinitis share type 2 inflammatory pathways. The Asthma Phenotypes in the Inner City (APIC) study reported rhinitis in 93.5% of 619 inner-city children with asthma and was more severe in those with difficult-to-control asthma6, consistent with other cohorts7, 8. In adults, allergic rhinitis severity correlates with asthma morbidity. Patients with persistent asthma and seasonal rhinitis have significantly greater asthma symptoms, hospitalizations, and cost of medical care than those without9–11.
Highly effective management strategies are available for allergic rhinitis, with intranasal corticosteroids (INCS) being the preferred monotherapy12 with adjunctive approaches including the addition of intranasal antihistamines, oral antihistamines, montelukast, and nasal saline irrigation12. The effect of rhinitis management on asthma control is less clear. A meta-analysis including 2162 patients found no significant change in asthma outcomes with the addition of INCS to orally inhaled corticosteroids13. A subsequent randomized controlled trial including 151 children with inadequately controlled asthma and chronic sinonasal disease found no differences in measures of impairment, FeNO, or methacholine reactivity with intranasal mometasone, but treatment did result in 36% fewer episodes of poor asthma control defined by 30% drops in peak expiratory flows for 2 consecutive days14.
Chronic Rhinosinusitis (with and without Nasal Polyps)
CRS can be present with (CRSwNP) and without nasal polyps (CRSsNP). CRSsNP is primarily a Th1/neutrophilic process whereas CRSwNP is a TH2/eosinophilic process11. Infection may play a role in the initiation or sustenance of inflammation in CRS15 and impact the lower airway by seeding chronic bronchitis, bronchiectasis, or inducing mucociliary dysfunction.
The cardinal symptoms of CRS include mucopurulent nasal discharge, nasal congestion or blockage, facial pain/pressure, and reduction or loss of sense of smell, as well as chronic cough16. Differentiating CRS from allergic and non-allergic rhinitis can be difficult16. Rhinoscopy is limited to visualization of the anterior structures of the nose whereas nasal endoscopy can identify purulent drainage from the inferior and middle meatus and sphenoethmoidal drainage, and polypoid tissue, but requires specific training and equipment not common to general clinical practice. Non-contrast sinus CT scan remains the preferred modality to image mucosal disease and occlusion of the sinus ostia17.
CRS is associated with impaired asthma control18 and increased exacerbation frequency19, and is an independent risk factor for difficult asthma20. Among the 709 Severe Asthma Research Program-3 (SARP-3) participants (including 187 children), the presence of chronic or recurrent sinusitis was associated with the exacerbation-prone (3+ exacerbations in the prior year) phenotype19.
Nasal polyposis is rare in children, regardless of underlying asthma, and should prompt a careful evaluation for other conditions, such as cystic fibrosis, ciliary dyskinesia, and aspirin exacerbated respiratory disease (AERD). Among the pediatric SARP-3 participants, fewer than 10% reported nasal polyposis and prevalence did not differ by asthma exacerbation frequency19. Data to support management of CRS in children with difficult-to-control asthma is very limited.
In adults, CRSwNP frequently indicates AERD. Histopathology demonstrates mast cell activation, marked eosinophilia, epithelial disruption, and pro-inflammatory cytokine production of both the upper and lower airways21. In such patients, aspirin desensitization has been shown to provide additional clinical benefit22.
Treatment of CRS includes oral and intranasal corticosteroids, antihistamines, nasal saline irrigation, and when associated with nasal polyposis, biologic therapies may be considered. Dupilumab, omalizumab, and mepolizumab are FDA approved therapies for CRSwNP and have been shown to lead to decreased polyp burden and improvement in quality of life23, 24. Reslizumab and benralizumab have also been studied but have not yet received FDA approval for CRSwNP. Preclinical data indicates that thymic stromal lymphopoietin (TSLP) may be upregulated in nasal polyp tissue in patients with CRSwNP25 suggesting tezepelumab, which binds TSLP, may be another potentially beneficial therapeutic in the pipeline26. Guidelines support consideration of biologic therapy in patients CRSwNP, with or without asthma27.
Retrospective analyses of patients undergoing endoscopic sinus surgery (ESS)/polypectomy have found ESS to improve asthma symptoms, improve FEV1, and decreased use of inhaled corticosteroids in both aspirin-sensitive and aspirin-tolerant patients28. Two recent meta-analyses of ESS in adults with asthma and chronic rhinosinusitis suggest modest benefits in clinical29 and pulmonary function30 outcomes, but the lack of high quality randomized controlled trials is limiting. Cost analysis, comparing ESS to treatment with dupilumab for CRSwNP, has demonstrated both produced quality adjusted life years (QALYs), but sinus surgery produced more QALYs and is less expensive than dupilumab31. However, in persons with comorbid difficult-to-control asthma, a biologic agent may improve both CRSwNP and asthma morbidity.
Obesity
Obesity, referring to excess body fat, is indirectly measured in clinical practice by body mass index (BMI), the anthropometric relationship between weight and height. In children, standard references are available from age 2 years32. The definition of overweight (BMI ≥ 85 percentile) and obese (BMI≥ 95 percentile) approach adult thresholds of BMI ≥ 25 and 30 kg/m2, respectively, in late adolescence.
Obesity and asthma have a bidirectional relationship33. Among children with asthma, obesity is associated with greater exacerbation frequency and severity, including need for mechanical ventilation34, greater symptom frequency, worse control35, poor quality of life36, impaired response to ICS therapy37, and worse parent-perceived overall child health38. Despite this, the vast majority of obese children hospitalized for asthma exacerbations lack a discharge diagnosis or treatment plans for obesity39.
Relatively small, randomized controlled trials have demonstrated beneficial effects of weight loss on asthma control and quality of life in children40–42. An 18-month multifactorial weight loss intervention demonstrated improvements in weight and asthma measures in both treatment and control groups, although lung function, asthma control and quality of life improved more in the intervention group42.
In adults, asthma and obesity are frequently comorbid43–49. Early onset allergic asthma (EOAA) develops in childhood, independent of obesity, and obesity can worsen asthma symptoms. Alternatively, late onset non-atopic asthma (LONA) may develop as a consequence of obesity, with the risk of asthma increasing by 50%50. LONA, characterized by a non–T2 phenotype, obesity, and female sex has been consistently identified in cluster analyses51–53.
Weight loss improves asthma-related quality of life, asthma control and lung function40, and in patients with LONA, asthma may resolve with weight loss54. A recent randomized trial of either dietary, exercise, or a combination of both interventions in overweight/obese adults with asthma found 5–10% weight loss resulted in clinically important improvements to asthma control in 58%, and quality of life in 83% of subjects55. Similarly, adding exercise to a short-term weight-loss program improved asthma control while achieving greater weight loss and aerobic capacity compared with weight loss alone56, 57. Bariatric surgery also improves asthma symptoms and exacerbation rates58–60, however, one study suggested improvements may be limited to those without metabolic syndrome61.
Inducible Laryngeal Obstruction
Inducible laryngeal obstruction (ILO), also called vocal cord dysfunction (VCD)62, paradoxical vocal fold movement disorder (PVFMD), or exercise-induced laryngeal obstruction (EILO)63, describes an episodic upper airway obstruction secondary to inappropriate narrowing of the true vocal fold and/or the supraglottic structures in response to external trigger. Exercise, irritants, and emotional stress are the primary inducers of ILO. ILO is associated with current or historical psychosocial disorders, particularly anxiety and stress64, 65, but also depression, post-traumatic stress disorder, and personality disorders, and others66. It can result in severe obstruction leading to intubation.
The prevalence of ILO among children with severe or difficult-to-control asthma is not well documented, but among adults with severe asthma, may be as high as 19–32%20, 67–69.Patients generally describe distinct episodes of dyspnea with an identifiable start and end, may have stridor as a prominent sign, and that are typically not prevented or resolved with beta-agonists. In the absence of symptoms, physical examination and spirometry are usually normal. Symptom questionnaires to detect ILO lack comprehensive validation70–72; while flattening of inspiratory flow-volume loop can be helpful but is neither sensitive nor specific73. The gold standard for diagnosis of ILO/EILO is continuous laryngoscopy during evoked challenge62.
Management of ILO and EILO involves speech-language therapy to allow patients to identify early symptoms and to employ controlled breathing techniques74. Behavioral interventions focus on avoidance of exposure to presumed triggers, visual biofeedback during episodes, and behavioral health/psychology62. Small prospective observational studies have reported reductions in asthma medication use and symptoms following these treatment strategies75, 76. Limited case series have reported inhaled anticholinergics may prevent symptoms77. Case reports have suggested benefit from tricyclic antidepressants, allergy or GERD therapies, inspiratory muscle training, botulinum toxin injections in the vocal folds78, 79, and in refractory cases, supraglottoplasty80.
Dysfunctional Breathing
Dysfunctional breathing describes disruption to the normal biomechanics of breathing and may present as hyperventilation, sighing, thoracic-dominant or thoraco-abdominal asynchronous patterns, among others, that leads to dyspnea and can mimic or be comorbid with difficult-to-control asthma in almost 50% of adults81, 82. It is associated with concurrent anxiety, depression and, to a lesser extent, sinonasal symptoms82. Instruments have been developed to accurately identify dysfunctional breathing83, 84 and retraining is associated with improved asthma control and quality of life85.
Obstructive Sleep Apnea (OSA)
Symptoms suggestive of obstructive sleep apnea (OSA) include snoring, gasping, choking, snorting during sleep, daytime hypersomnolence, morning headaches, and particularly in children, behavioral disturbances and inattentiveness. Physical examination may identify obesity and/or anatomically narrowed oropharyngeal airway. The gold standard for diagnosis in both adults and children is polysomnography.
In children, asthma is a risk factor for more severe OSA and OSA is associated with more severe asthma86. Abnormal scores on the Pediatric Sleep Questionnaire (PSQ), a validated screening tool for OSA, are associated with poor asthma control87, 88. Adenotonsillectomy is the first line treatment for OSA in children, which can result in significant improvements in asthma exacerbations89 and symptom control86.
In adults, obesity is the major risk factor linking asthma and OSA, and this can be augmented by frequent oral corticosteroid use resulting in myopathy, weight gain, and fat redistribution to the neck area. Independent of OCS, inhaled corticosteroids (ICS) are associated with habitual snoring and OSA in a dose-dependent manner90. Prospective studies demonstrate an increased risk of incident OSA among patients with asthma91–94, however, the relationship to asthma severity is inconsistent. Some studies report increased OSA in severe compared to moderate asthma90, 95–97 while others find no difference98, 99. Similarly, OSA is associated with increased asthma exacerbations, decreased quality of life and asthma control in some studies100, 101, while others report no relationship to asthma morbidity20, 96.
Continuous positive airway pressure (CPAP), the primary intervention for adult OSA, has been associated with improved quality of life102, improved asthma control102, 103 and decreased decline in FEV195. However, a recent meta-analysis found CPAP improved quality of life but has an inconsistent impact on asthma control and lung function104.
Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) typically presents with heartburn and food regurgitation, but chest pain, odynophagia, and dysphagia may also occur.
Extraesophageal symptoms of chronic cough, hoarseness and wheezing105–108 may be due to neuronally-mediated bronchial hyperresponsiveness, airway edema, mucus secretion105, alterations of mucosal immunity109, and micro-aspiration. Diagnosis is often based on the presence of classic symptoms of heartburn. A trial of lifestyle and diet modification, and acid suppression therapy may be pursued prior to invasive testing. Additional evaluation may include upper gastrointestinal endoscopy which can help differentiate GERD from eosinophilic esophagitis and alternative diagnoses, and assess complications, such as strictures mucosal metaplasia, dysplasia and malignancy in adults. Ambulatory pH or impedance monitoring of the esophagus in concert with symptom recording can identify frequency of reflux events and correlate with symptoms to aid in diagnosis110, 111.
In children, GERD is associated with 36% increased odds of uncontrolled asthma. The SARP-3 cohort found GERD was associated with exacerbation prone asthma in both children and adults19. Despite these associations, a randomized double blind placebo control study evaluating the effect of lansoprazole in children with poorly controlled asthma and asymptomatic GERD failed to show a change in asthma control, lung function, quality of life or acute exacerbations. The treatment group reported more upper respiratory infections, sore throat, and bronchitis, raising potential safety concerns112.
GERD is present in 60–80% of adults with asthma. However, clinical trials of anti-reflux therapy have demonstrated no significant effect on asthma control or exacerbations113, 114, even among those with positive esophageal pH probe studies115. A recent Cochrane review of adult and pediatric studies concluded there was uncertain evidence that medical treatment of GERD improved exacerbations though reported moderate evidence for slightly improved FEV1 (O.1 L, 95% CI 0.05 to 0.15) and β-agonist use (−0.7 puffs/day, 95% CI −1.20 to −0.22)116. For both pediatric and adult patients with difficult-to-treat asthma, a trial of anti-reflux therapy should be reserved only for patients with symptomatic reflux117, 118. Surgical therapy has insufficient evidence for recommendation, though one randomized trial of fundoplication reported improvement in asthma symptoms119.
Anxiety/depression
Pateraki, Vance and Morris describe three potential mechanisms for comorbid anxiety and asthma: asthma triggering unhelpful thinking and behavior that raises anxiety; anxiety impairing self-care and triggering hyperventilation; and both leading to self-perpetuating feedback cycles and symptom confusion38. Anxiety is also associated with altered perception of dyspnea and greater symptom reporting36. Physiologic responses to psychological distress include decreased expression of beta-2-adrenergic receptor (ADRB2) and glucocorticoid receptor NR3C1120, 121, enhanced generation of pro-inflammatory cytokines122, and stress-induced changes in gene expression that regulate immunological responses118, 121, 123.
Children with asthma have a higher prevalence of anxiety and depression which correlates with asthma severity124–127; up to 60% of children with severe asthma express anxious symptomatology128, 129. Children with anxiety report more asthma symptoms130, have lower ACT scores, more frequent exacerbations35, longer length of hospital stay for asthma131, and greater use of rescue medications. In a large pediatric claims-based study, Bardach et al. found an increased rate of emergency department (ED) visits for asthma in patients with anxiety or depression, and greatest for those with both conditions132. Additionally, psychological stress of the child’s caregiver is associated with greater asthma symptoms133 and ED utilization134–136. Depressed mothers are more likely to report medication non-adherence, lack of understanding of the medications’ functions, and inability to manage episodes at home137.
In adults, epidemiologic evidence suggests a relationship between anxiety and depression with asthma and allergic diseases138. Anxiety and depression are related to worse asthma control, exacerbations, and healthcare utilization. Up to 24% of asthma patients report anxiety and 12% report depression, and these patients have a significant increase in exacerbations and use of health care resources, with a four-fold greater impact of anxiety on asthma control than depression139.
Most evidence supports cognitive behavioral therapy can improve anxiety in children and adults with asthma, but few data support improved asthma outcomes140–143. A trial of antidepressive medication may improve symptoms and exacerbations in adults with high levels of asthma morbidity and major depressive symptoms144.
Vitamin D deficiency
Hypovitaminosis D leading to osteomalacia or rickets in children is rare in developed parts of the world, but prevalence of vitamin D <20 ng/ml [50 nmol/L] is approximately 15% for children145 and 18% in US adults146. It is typically assessed by serum 25-hydroxyvitamin D (25[OH]D).
Vitamin D deficiency is associated with impaired lung function147, increased exacerbation frequency148, severity149, and reduced corticosteroid responsiveness150, 151 in asthmatics. It has been associated with lower mean FEV1 and increased odds of severe asthma exacerbations in the Childhood Asthma Management Program (CAMP) and other pediatric cohorts151–153 and correlates with increased need for inhaled and oral corticosteroids to achieve asthma control154.
Evidence that vitamin D supplementation leads to improvement in asthma control is inconsistent117. Recent systematic reviews have demonstrated that vitamin D supplementation in children and adults reduces the rate of asthma exacerbations and risk of ED visit or hospitalization in those with low vitamin D155, 156. However, the recent Vitamin D to Prevent Severe Asthma Exacerbations (VDKA) Study ended prior to full enrollment but found no benefit to supplemental vitamin D in children who were deficient157. The Vitamin D Add-On Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA) study determined that supplemental oral vitamin D3 added to inhaled corticosteroids did not alter the rate of first treatment failure or reduce exacerbations in adults with persistent asthma and vitamin D deficiency. Notably, the subset of patients that achieved vitamin D sufficiency (>30 ng/ml) had significant reduction in exacerbations158.
Allergic bronchopulmonary aspergillosis/Severe Asthma with Fungal Sensitization
Allergic bronchopulmonary aspergillosis (ABPA) refers to a hypersensitivity reaction to Aspergillus fumigatus, though a similar syndrome exists for a variety of molds, termed allergic bronchopulmonary mycosis, ABPM. It is hypothesized that the presence of Aspergillus antigens from fungal spore inhalation leads to a hypersensitivity immune response associated with inflammation of the airways in susceptible hosts159, 160.
ABPA typically presents as uncontrolled asthma or chronic bronchitis symptoms with or without the expectoration of mucus plugs. Diagnostic criteria include evidence of an immune reaction to aspergillus in patients with predisposing asthma or cystic fibrosis (see table 2)161. High resolution chest CT may identify central bronchiectasis.
The prevalence of ABPA in patients with asthma ranges from 2.5% to 22.3%, with a pooled prevalence of 8.4%162, 163. It is more common in adults164. A recent study at a single site in India found 11% of children with poorly controlled asthma had comorbid ABPA and 61% had aspergillus sensitivity. Fungal sensitization, alone, has been associated with severe asthma classification and lower lung function165.
Management of ABPA includes systemic corticosteroids166 and adjunctive antifungal therapy. Itraconazole is the only antifungal agent evaluated in randomized controlled trials167 but small studies have used voriconazole and posaconazole. Experience with anti-IgE therapy, omalizumab, is growing in ABPA but few randomized controlled trials exist; a synthesis of the published data reported significant reductions in exacerbations, improved asthma control and lung function in patients with ABPA168. Anti-IL5/-IL5R169–171 and anti-IL4R172 have been effective in cases refractory to corticosteroids, antifungals and omalizumab.
More recently, a phenotype of severe asthma with fungal sensitization (SAFS) has been defined as severe asthma and sensitization to a fungal species without evidence of ABPA or ABPM160, likely part of a spectrum of fungal sensitization-induced airways reaction159. Randomized controlled trials of antifungal medications for SAFS have reported conflicting results. A randomized controlled trial of itraconazole in adults led to improved asthma quality of life and peak flow measurements173. However, a 3-month intervention with voriconazole failed to find improved exacerbation rates or quality of life174. The American Thoracic Society/European Respiratory Society Taskforce on Severe asthma discourage use antifungal agents for the treatment of severe asthma outside of ABPA175.
Asthmatic smokers/ACO
Smoking in adolescence has been associated with asthma symptoms and airflow obstruction in general population studies176. Adolescents with asthma may have higher rates of nicotine dependence, are more likely to use E-cigarettes177, and more difficulty achieving smoking cessation178. Secondhand smoke (SHS) exposure in children with asthma is associated with greater asthma symptoms179, medication use180, ED visits181, and impaired lung function182–184. Interventions to decrease SHS improve asthma severity and lung function185. Asthma guidelines universally recommend smoking cessation and SHS avoidance118, 186.
Adult asthmatics who smoke have worse asthma control, lung function decline, and response to asthma medications187. The mechanism is thought to be related to increased airway permeability and relative corticosteroid resistance188, 189. Price et al randomized 1,019 patients to montelukast 10mg/day, fluticasone propionate 250 mcg twice daily or placebo and found that both treatments significantly increased the mean days of asthma control with a greater benefit with fluticasone in those with ≤11 pack-year smoking history and greater benefit with montelukast in those with greater smoking history190. Therefore, in addition to smoking cessation interventions, clinicians should assess smoking history to decide whether to treat with inhaled corticosteroids or montelukast.
The combination of asthma and chronic obstructive pulmonary disease (COPD), referred to as ACO, is a distinct phenotype associated with more exacerbations, worse quality of life and increased healthcare costs191. Pharmacotherapy should be based on the clinical features favoring asthma or COPD along the spectrum of airway disease186, 192. For example, for features favoring asthma, low dose inhaled corticosteroids are indicated with addition of LABA or LAMA, as needed; if features favor COPD, then start with bronchodilators. Scant evidence exists for the use of a PDE inhibitor, such as roflumilast193.
Hormonal fluctuation
Approximately one third of reproductive age women experience changes of ovarian hormones during the menstrual cycle that have been linked to increased symptoms and decreased peak flow rates194, 195, correlating with increase sputum eosinophils and FeNO during the premenstrual phase196. Women with perimenstrual worsening of asthma also report greater urgent health-care utilization for asthma197, though emergency department-based studies have not found variation in menstrual phase among women presenting with asthma exacerbations198, 199. Animal models support the role of estrogen-dependent mechanisms that augment allergen-mediated airway inflammation and airway hyperreactivity200. Cross-sectional observational studies investigating oral contraceptive use on asthma morbidity have had mixed results201, 202. Randomized controlled interventional trials in patients with perimenstrual worsening of asthma are needed.
During pregnancy, about 1/3 of women have worsened asthma, with similar proportions having improvement and no change in asthma symptoms203. Increased asthma severity is associated with gestational asthma exacerbations204. However, adherence to asthma medication substantially improves the risk of worsened asthma symptoms during pregnancy205, 206.
Addressing comorbidities in clinical practice
Evaluating adult and pediatric patients with difficult-to-treat asthma involves confirming the appropriate diagnosis, ensuring proper medication adherence and delivery, minimizing environmental triggers, and assessing and optimally managing comorbid conditions. It is important to consider comorbidities early in the evaluation and periodically reassess for their development and impact. A detailed history and physical examination provide the clinical clues to guide further evaluation for most of the comorbid conditions reviewed here (Figure 1). Testing for allergic sensitization can be helpful in assessing both asthma endotypes and atopic comorbidities. Laboratory evaluation including total IgE and vitamin D level may be used to assess biologic drug eligibility as well as screen for the possibility of ABPA, and vitamin D deficiency. Failure to improve nasal obstruction with appropriate therapy, or in the absence of atopy, should lead to further evaluation for CRS. A total serum IgE >1000 IU/ml raises suspicion for ABPA and should prompt further evaluation. As the timing and specific items evaluated will depend on individual patient characteristics, presenting signs and clinical course, there is no simple algorithm that can be utilized to systematically evaluate all of them. Table 2 details clinical clues and appropriate evaluation and management considerations for each asthma comorbidity.
Figure 1. Flow diagram for recommended evaluation of comorbidities in patients with Difficult to Control asthma.
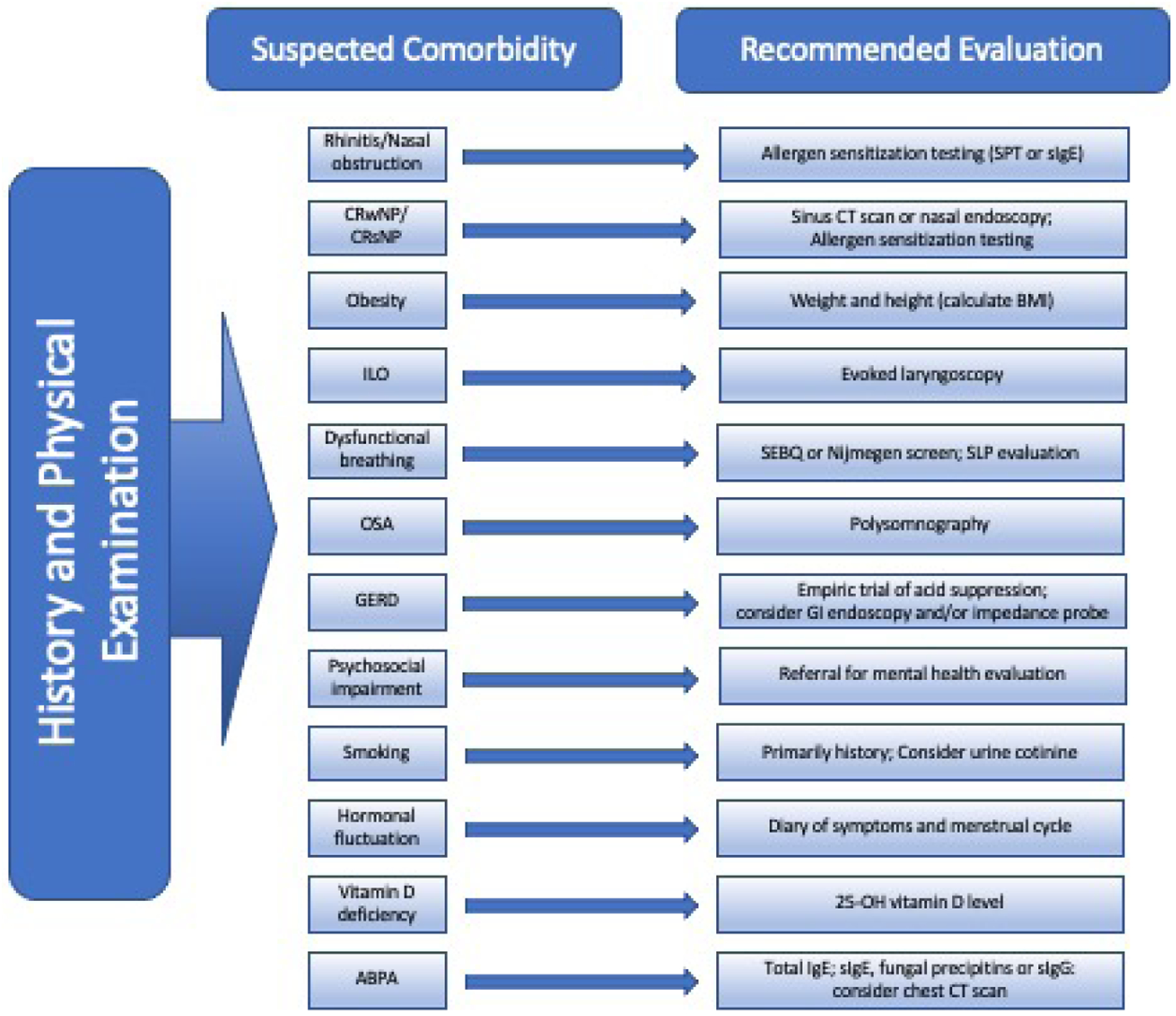
CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps;ILO, inducible laryngeal obstruction; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux; SPT, skin prick testing; sIgE, specific immunoglobulin E; CT, computed tomography; BMI, body mass index; SEBQ, Self-Evaluation of Breathing Questionnaire; GI, gastroenterology; PSG, polysomnography; ABPA, allergic bronchopulmonary aspergillosis; IgE, immunoglobulin E; sIgG, specific Immunoglobulin G.
Knowledge gaps
Further research is needed to understand the interaction of multiple comorbidities on asthma morbidity. For example, children who are overweight or obese have the greatest symptoms with smoke exposure207. Knowing the synergistic or effect-modifying effects of multi-comorbidity on asthma outcomes will help prioritize interventions. Broadening the evidentiary basis for treatment outcomes in asthma is greatly needed for most of the identified comorbidities. Large-scale randomized controlled clinical trials to test the effect of management of the comorbid condition(s) on asthma will enhance the personalized medicine approach to patient care. Moreover, further understanding how available and future therapies may differentially affect asthmatic populations with comorbidities will help clinicians select optimal treatments for their patients.
Conclusions
Identifying asthma comorbidities is an essential element of the evaluation of difficult-to-control asthma in children and adults. Targeted interventions to smoking cessation, obesity, OSA, ABPA and CRSwNP are likely to improve asthma morbidity. Treating allergic rhinitis, symptomatic GERD, vitamin D deficiency, and anxiety/depression should be considered to improve the comorbid condition but lack clear evidence for improving asthma. For patients in whom a monoclonal antibody-based therapy is considered, evaluation for concurrent chronic rhinosinusitis with nasal polyposis and allergic sensitization may inform a selection beneficial to both asthma and the comorbid condition.
Conflict of interest statement for authors:
Dr. Gaffin receives grants funding from NIH and Boston Children’s Hospital. He receives personal fees from Syneos Health and Answers in CME, outside the submitted work. Dr. Castro receives University Grant Funding from NIH, American Lung Association, PCORI. He receives Pharmaceutical Grant Funding from AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, Shionogi. He is a consultant for Genentech, Teva, Sanofi-Aventis, Novartis. He is a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva. He receives Royalties from Elsevier.
Dr. Bacharier reports personal fees from GlaxoSmithKline Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura, Circassia, Elsevier, Kinaset, and Vertex outside the submitted work.
Dr Fuhlbrigge is an unpaid consultant to AstraZeneca for the development of outcome measures for asthma and COPD clinical trials and a consultant to Novartis on epidemiologic analyses related to asthma control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corren J Allergic rhinitis and asthma: how important is the link? J Allergy Clin Immunol 1997; 99:S781–6. [DOI] [PubMed] [Google Scholar]
- 2.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002; 109:419–25. [DOI] [PubMed] [Google Scholar]
- 3.Greisner WA 3rd, Settipane RJ, Settipane GA. Co-existence of asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Asthma Proc 1998; 19:185–8. [DOI] [PubMed] [Google Scholar]
- 4.Huovinen E, Kaprio J, Laitinen LA, Koskenvuo M. Incidence and prevalence of asthma among adult Finnish men and women of the Finnish Twin Cohort from 1975 to 1990, and their relation to hay fever and chronic bronchitis. Chest 1999; 115:928–36. [DOI] [PubMed] [Google Scholar]
- 5.Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 2012; 26:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Togias A, Gergen PJ, Hu JW, Babineau DC, Wood RA, Cohen RT, et al. Rhinitis in children and adolescents with asthma: Ubiquitous, difficult to control, and associated with asthma outcomes. J Allergy Clin Immunol 2019; 143:1003–11 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arabkhazaeli A, Vijverberg SJ, van Erp FC, Raaijmakers JA, van der Ent CK, Maitland van der Zee AH. Characteristics and severity of asthma in children with and without atopic conditions: a cross-sectional study. BMC Pediatr 2015; 15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016; 138:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet J, Boushey HA, Busse WW, Canonica GW, Durham SR, Irvin CG, et al. Characteristics of patients with seasonal allergic rhinitis and concomitant asthma. Clin Exp Allergy 2004; 34:897–903. [DOI] [PubMed] [Google Scholar]
- 10.Yawn BP, Yunginger JW, Wollan PC, Reed CE, Silverstein MD, Harris AG. Allergic rhinitis in Rochester, Minnesota residents with asthma: frequency and impact on health care charges. J Allergy Clin Immunol 1999; 103:54–9. [DOI] [PubMed] [Google Scholar]
- 11.Halpern MT, Schmier JK, Richner R, Guo C, Togias A. Allergic rhinitis: a potential cause of increased asthma medication use, costs, and morbidity. J Asthma 2004; 41:117–26. [DOI] [PubMed] [Google Scholar]
- 12.Dykewicz MS, Wallace DV, Amrol DJ, Baroody FM, Bernstein JA, Craig TJ, et al. Rhinitis 2020: A practice parameter update. J Allergy Clin Immunol 2020; 146:721–67. [DOI] [PubMed] [Google Scholar]
- 13.Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy 2013; 68:569–79. [DOI] [PubMed] [Google Scholar]
- 14.American Lung Association-Asthma Clinical Research Centers’ Writing C, Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. J Allergy Clin Immunol 2015; 135:701–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012; 50:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020; 58:1–464. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 2015; 152:S1–s39. [DOI] [PubMed] [Google Scholar]
- 18.Dixon AE, Kaminsky DA, Holbrook JT, Wise RA, Shade DM, Irvin CG. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest 2006; 130:429–35. [DOI] [PubMed] [Google Scholar]
- 19.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med 2017; 195:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay TR, Radhakrishna N, Hore-Lacy F, Smith C, Hoy R, Dabscheck E, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016; 21:1384–90. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol 2006; 118:773–86; quiz 87–8. [DOI] [PubMed] [Google Scholar]
- 22.Stevens WW, Jerschow E, Baptist AP, Borish L, Bosso JV, Buchheit KM, et al. The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin-exacerbated respiratory disease: A Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2021; 147:827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394:1638–50. [DOI] [PubMed] [Google Scholar]
- 24.Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020; 146:595–605. [DOI] [PubMed] [Google Scholar]
- 25.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2013; 132:593–600.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy US, Melder K, Patel VA, Lee SE. Current Evidence for Biologic Therapy in Chronic Rhinosinusitis with Nasal Polyposis. Otolaryngol Clin North Am 2021; 54:689–99. [DOI] [PubMed] [Google Scholar]
- 27.Fokkens WJ, Lund V, Bachert C, Mullol J, Bjermer L, Bousquet J, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019; 74:2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awad OG, Fasano MB, Lee JH, Graham SM. Asthma outcomes after endoscopic sinus surgery in aspirin-tolerant versus aspirin-induced asthmatic patients. Am J Rhinol 2008; 22:197–203. [DOI] [PubMed] [Google Scholar]
- 29.Vashishta R, Soler ZM, Nguyen SA, Schlosser RJ. A systematic review and meta-analysis of asthma outcomes following endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol 2013; 3:788–94. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Hong H, Sun Y, Lai Y, Xu R, Shi J, et al. The effects of endoscopic sinus surgery on pulmonary function in chronic rhinosinusitis patients with asthma: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2019; 276:1405–11. [DOI] [PubMed] [Google Scholar]
- 31.Scangas GA, Wu AW, Ting JY, Metson R, Walgama E, Shrime MG, et al. Cost Utility Analysis of Dupilumab Versus Endoscopic Sinus Surgery for Chronic Rhinosinusitis With Nasal Polyps. Laryngoscope 2021; 131:E26–e33. [DOI] [PubMed] [Google Scholar]
- 32.CDC Growth Charts. CDC.gov: Centers for Disease Control and Prevention.] Available from https://www.cdc.gov/growthcharts/clinical_charts.htm.
- 33.Shan LS, Zhou QL, Shang YX. Bidirectional Association Between Asthma and Obesity During Childhood and Adolescence: A Systematic Review and Meta-Analysis. Front Pediatr 2020; 8:576858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo Y, Nochioka K, Hataya H, Sakakibara H, Terakawa T, Testa M. Burden of Obesity on Pediatric Inpatients with Acute Asthma Exacerbation in the United States. J Allergy Clin Immunol Pract 2016; 4:1227–31. [DOI] [PubMed] [Google Scholar]
- 35.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med 2013; 187:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol 2007; 119:591–6. [DOI] [PubMed] [Google Scholar]
- 37.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol 2011; 127:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong H, Hou W, Kaur S, Bianchi-Hayes JM. The association of co-morbid asthma and overweight/obese status with healthcare utilization and caregiver perception of health in children 4–17 years, a NHANES study. J Asthma 2021:1–7. [DOI] [PubMed] [Google Scholar]
- 39.Borgmeyer A, Ercole PM, Niesen A, Strunk RC. Lack of Recognition, Diagnosis, and Treatment of Overweight/Obesity in Children Hospitalized for Asthma. Hosp Pediatr 2016; 6:667–76. [DOI] [PubMed] [Google Scholar]
- 40.Okoniewski W, Lu KD, Forno E. Weight Loss for Children and Adults with Obesity and Asthma. A Systematic Review of Randomized Controlled Trials. Ann Am Thorac Soc 2019; 16:613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, Elizalde-Lozano AM. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int Arch Allergy Immunol 2014; 163:252–8. [DOI] [PubMed] [Google Scholar]
- 42.Willeboordse M, van de Kant KDG, Tan FE, Mulkens S, Schellings J, Crijns Y, et al. A Multifactorial Weight Reduction Programme for Children with Overweight and Asthma: A Randomized Controlled Trial. PLoS One 2016; 11:e0157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018; 141:1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax 1999; 54:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camargo CA Jr., Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 1999; 159:2582–8. [DOI] [PubMed] [Google Scholar]
- 46.Beckett WS, Jacobs DR Jr., Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 2001; 164:2045–50. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 2002; 155:191–7. [DOI] [PubMed] [Google Scholar]
- 48.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002; 122:1256–63. [DOI] [PubMed] [Google Scholar]
- 49.Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. Eur Respir J 2004; 24:740–4. [DOI] [PubMed] [Google Scholar]
- 50.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefaudeux D, De Meulder B, Loza MJ, Peffer N, Rowe A, Baribaud F, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol 2017; 139:1797–807. [DOI] [PubMed] [Google Scholar]
- 53.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates JHT, Poynter ME, Frodella CM, Peters U, Dixon AE, Suratt BT. Pathophysiology to Phenotype in the Asthma of Obesity. Ann Am Thorac Soc 2017; 14:S395–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013; 43:36–49. [DOI] [PubMed] [Google Scholar]
- 56.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am J Respir Crit Care Med 2017; 195:32–42. [DOI] [PubMed] [Google Scholar]
- 57.Kolinsky NC, Weare-Regales N, Lockey RF. A Practical Approach to Assist Asthmatics to Lose Weight. J Allergy Clin Immunol Pract 2021; 9:2245–54. [DOI] [PubMed] [Google Scholar]
- 58.Dixon JB, Chapman L, O’Brien P. Marked improvement in asthma after Lap-Band surgery for morbid obesity. Obes Surg 1999; 9:385–9. [DOI] [PubMed] [Google Scholar]
- 59.Dhabuwala A, Cannan RJ, Stubbs RS. Improvement in co-morbidities following weight loss from gastric bypass surgery. Obes Surg 2000; 10:428–35. [DOI] [PubMed] [Google Scholar]
- 60.Macgregor AM, Greenberg RA. Effect of Surgically Induced Weight Loss on Asthma in the Morbidly Obese. Obes Surg 1993; 3:15–21. [DOI] [PubMed] [Google Scholar]
- 61.Forno E, Zhang P, Nouraie M, Courcoulas A, Mitchell JE, Wolfe BM, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS One 2019; 14:e0214730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halvorsen T, Walsted ES, Bucca C, Bush A, Cantarella G, Friedrich G, et al. Inducible laryngeal obstruction: an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J 2017; 50. [DOI] [PubMed] [Google Scholar]
- 63.Olin JT, Clary MS, Fan EM, Johnston KL, State CM, Strand M, et al. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J 2016; 48:1192–200. [DOI] [PubMed] [Google Scholar]
- 64.Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med 2013; 178:1208–12. [DOI] [PubMed] [Google Scholar]
- 65.Gavin LA, Wamboldt M, Brugman S, Roesler TA, Wamboldt F. Psychological and family characteristics of adolescents with vocal cord dysfunction. J Asthma 1998; 35:409–17. [DOI] [PubMed] [Google Scholar]
- 66.Husein OF, Husein TN, Gardner R, Chiang T, Larson DG, Obert K, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope 2008; 118:740–7. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Denton E, Hoy R, Tay TR, Bondarenko J, Hore-Lacy F, et al. Paradoxical Vocal Fold Motion in Difficult Asthma Is Associated with Dysfunctional Breathing and Preserved Lung Function. J Allergy Clin Immunol Pract 2020; 8:2256–62. [DOI] [PubMed] [Google Scholar]
- 68.Lee JW, Tay TR, Paddle P, Richards AL, Pointon L, Voortman M, et al. Diagnosis of concomitant inducible laryngeal obstruction and asthma. Clin Exp Allergy 2018; 48:1622–30. [DOI] [PubMed] [Google Scholar]
- 69.Low K, Lau KK, Holmes P, Crossett M, Vallance N, Phyland D, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med 2011; 184:50–6. [DOI] [PubMed] [Google Scholar]
- 70.Fowler SJ, Thurston A, Chesworth B, Cheng V, Constantinou P, Vyas A, et al. The VCDQ--a Questionnaire for symptom monitoring in vocal cord dysfunction. Clin Exp Allergy 2015; 45:1406–11. [DOI] [PubMed] [Google Scholar]
- 71.Traister RS, Fajt ML, Landsittel D, Petrov AA. A novel scoring system to distinguish vocal cord dysfunction from asthma. J Allergy Clin Immunol Pract 2014; 2:65–9. [DOI] [PubMed] [Google Scholar]
- 72.Gartner-Schmidt JL, Shembel AC, Zullo TG, Rosen CA. Development and validation of the Dyspnea Index (DI): a severity index for upper airway-related dyspnea. J Voice 2014; 28:775–82. [DOI] [PubMed] [Google Scholar]
- 73.Christensen PM, Maltbæk N, Jørgensen IM, Nielsen KG. Can flow-volume loops be used to diagnose exercise induced laryngeal obstructions? A comparison study examining the accuracy and inter-rater agreement of flow volume loops as a diagnostic tool. Prim Care Respir J 2013; 22:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barker N, Thevasagayam R, Ugonna K, Kirkby J. Pediatric Dysfunctional Breathing: Proposed Components, Mechanisms, Diagnosis, and Management. Front Pediatr 2020; 8:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kramer S, deSilva B, Forrest LA, Matrka L. Does treatment of paradoxical vocal fold movement disorder decrease asthma medication use? Laryngoscope 2017; 127:1531–7. [DOI] [PubMed] [Google Scholar]
- 76.Ivancic R, Matrka L, Wiet G, Puckett A, Haney J, deSilva B. Reduced Asthma Medication Use after Treatment of Pediatric Paradoxical Vocal Fold Motion Disorder. Laryngoscope 2021; 131:1639–46. [DOI] [PubMed] [Google Scholar]
- 77.Doshi DR, Weinberger MM. Long-term outcome of vocal cord dysfunction. Ann Allergy Asthma Immunol 2006; 96:794–9. [DOI] [PubMed] [Google Scholar]
- 78.Montojo J, González R, Hernández E, Zafra M, Plaza G. Office-based laryngeal injection of botulinum toxin for paradoxical vocal fold motion in a child. Int J Pediatr Otorhinolaryngol 2015; 79:1161–3. [DOI] [PubMed] [Google Scholar]
- 79.Cheng YS, Bhutta MF, Ramsden JD, Lennox P. Periodic botulinum toxin injections for paradoxical vocal fold motion in a child with cerebral palsy: a case study. Int J Pediatr Otorhinolaryngol 2014; 78:570–1. [DOI] [PubMed] [Google Scholar]
- 80.Norlander K, Johansson H, Jansson C, Nordvall L, Nordang L. Surgical treatment is effective in severe cases of exercise-induced laryngeal obstruction: A follow-up study. Acta Otolaryngol 2015; 135:1152–9. [DOI] [PubMed] [Google Scholar]
- 81.Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016; 25:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denton E, Bondarenko J, Tay T, Lee J, Radhakrishna N, Hore-Lacy F, et al. Factors Associated with Dysfunctional Breathing in Patients with Difficult to Treat Asthma. J Allergy Clin Immunol Pract 2019; 7:1471–6. [DOI] [PubMed] [Google Scholar]
- 83.van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985; 29:199–206. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell AJ, Bacon CJ, Moran RW. Reliability and Determinants of Self-Evaluation of Breathing Questionnaire (SEBQ) Score: A Symptoms-Based Measure of Dysfunctional Breathing. Appl Psychophysiol Biofeedback 2016; 41:111–20. [DOI] [PubMed] [Google Scholar]
- 85.Denton E, Bondarenko J, O’Hehir RE, Hew M. Breathing pattern disorder in difficult asthma: Characteristics and improvement in asthma control and quality of life after breathing re-training. Allergy 2019; 74:201–3. [DOI] [PubMed] [Google Scholar]
- 86.Sanchez T, Castro-Rodriguez JA, Brockmann PE. Sleep-disordered breathing in children with asthma: a systematic review on the impact of treatment. J Asthma Allergy 2016; 9:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dooley AA, Jackson JH, Gatti ML, Fanous H, Martinez C, Prue DC, et al. Pediatric sleep questionnaire predicts more severe sleep apnea in children with uncontrolled asthma. J Asthma 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gunnlaugsson S, Abul MH, Wright L, Petty CR, Permaul P, Gold DR, et al. Associations of Snoring and Asthma Morbidity in the School Inner-City Asthma Study. J Allergy Clin Immunol Pract 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhattacharjee R, Choi BH, Gozal D, Mokhlesi B. Association of adenotonsillectomy with asthma outcomes in children: a longitudinal database analysis. PLoS Med 2014; 11:e1001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest 2009; 135:1125–32. [DOI] [PubMed] [Google Scholar]
- 91.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest 2006; 130:1779–83. [DOI] [PubMed] [Google Scholar]
- 92.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. Jama 2015; 313:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen TC, Lin CL, Wei CC, Chen CH, Tu CY, Hsia TC, et al. Risk of Obstructive Sleep Apnea in Adult Patients with Asthma: A Population-Based Cohort Study in Taiwan. PLoS One 2015; 10:e0128461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kong DL, Qin Z, Shen H, Jin HY, Wang W, Wang ZF. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Sci Rep 2017; 7:4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang TY, Lo YL, Lin SM, Huang CD, Chung FT, Lin HC, et al. Obstructive sleep apnoea accelerates FEV(1) decline in asthmatic patients. BMC Pulm Med 2017; 17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol 2009; 124:371–6. [DOI] [PubMed] [Google Scholar]
- 97.Teodorescu M, Polomis DA, Gangnon RE, Fedie JE, Consens FB, Chervin RD, et al. Asthma Control and Its Relationship with Obstructive Sleep Apnea (OSA) in Older Adults. Sleep Disord 2013; 2013:251567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teodorescu M, Broytman O, Curran-Everett D, Sorkness RL, Crisafi G, Bleecker ER, et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract 2015; 3:566–75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med 2008; 9:494–9. [DOI] [PubMed] [Google Scholar]
- 100.Kim MY, Jo EJ, Kang SY, Chang YS, Yoon IY, Cho SH, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol 2013; 110:253–7, 7.e1. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Liu K, Hu K, Yang J, Li Z, Nie M, et al. Impact of obstructive sleep apnea on severe asthma exacerbations. Sleep Med 2016; 26:1–5. [DOI] [PubMed] [Google Scholar]
- 102.Serrano-Pariente J, Plaza V, Soriano JB, Mayos M, López-Viña A, Picado C, et al. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 2017; 72:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kauppi P, Bachour P, Maasilta P, Bachour A. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep Breath 2016; 20:1217–24. [DOI] [PubMed] [Google Scholar]
- 104.Davies SE, Bishopp A, Wharton S, Turner AM, Mansur AH. Does Continuous Positive Airway Pressure (CPAP) treatment of obstructive sleep apnoea (OSA) improve asthma-related clinical outcomes in patients with co-existing conditions?- A systematic review. Respir Med 2018; 143:18–30. [DOI] [PubMed] [Google Scholar]
- 105.de Benedictis FM, Bush A. Respiratory manifestations of gastro-oesophageal reflux in children. Arch Dis Child 2018; 103:292–6. [DOI] [PubMed] [Google Scholar]
- 106.Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102. [DOI] [PubMed] [Google Scholar]
- 107.Tucci F, Resti M, Fontana R, Novembre E, Lami CA, Vierucci A. Gastroesophageal reflux and bronchial asthma: prevalence and effect of cisapride therapy. J Pediatr Gastroenterol Nutr 1993; 17:265–70. [DOI] [PubMed] [Google Scholar]
- 108.Cinquetti M, Micelli S, Voltolina C, Zoppi G. The pattern of gastroesophageal reflux in asthmatic children. J Asthma 2002; 39:135–42. [DOI] [PubMed] [Google Scholar]
- 109.Hait EJ, McDonald DR. Impact of Gastroesophageal Reflux Disease on Mucosal Immunity and Atopic Disorders. Clin Rev Allergy Immunol 2019; 57:213–25. [DOI] [PubMed] [Google Scholar]
- 110.Katz PO, Gerson LB, Vela MF. Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease. Official journal of the American College of Gastroenterology | ACG; 2013; 108:308–28. [DOI] [PubMed] [Google Scholar]
- 111.Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2018; 66:516–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. Jama 2012; 307:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Good JT Jr., Kolakowski CA, Groshong SD, Murphy JR, Martin RJ. Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest 2012; 141:599–606. [DOI] [PubMed] [Google Scholar]
- 114.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360:1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dixon AE, Clerisme-Beaty EM, Sugar EA, Cohen RI, Lang JE, Brown ED, et al. Effects of obstructive sleep apnea and gastroesophageal reflux disease on asthma control in obesity. J Asthma 2011; 48:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kopsaftis Z, Yap HS, Tin KS, Hnin K, Carson-Chahhoud KV. Pharmacological and surgical interventions for the treatment of gastro-oesophageal reflux in adults and children with asthma. Cochrane Database Syst Rev 2021; 5:Cd001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.“Global strategy for asthma management and prevention: GINA executive summary.” Bateman ED, Hurd SS, Barnes PJ, Bousquet, Drazen JM, FitzGerald JM, Gibson P, Ohta K, O’Byrne P, Pedersen SE, Pizzichini, Sullivan SD, Wenzel SE and Zar HJ. Eur Respir J 2008; 31: 143–178. Eur Respir J 2018; 51. [DOI] [PubMed] [Google Scholar]
- 118.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120:S94–138. [DOI] [PubMed] [Google Scholar]
- 119.Koch OO, Antoniou SA, Kaindlstorfer A, Asche KU, Granderath FA, Pointner R. Effectiveness of laparoscopic total and partial fundoplication on extraesophageal manifestations of gastroesophageal reflux disease: a randomized study. Surg Laparosc Endosc Percutan Tech 2012; 22:387–91. [DOI] [PubMed] [Google Scholar]
- 120.Brehm JM, Ramratnam SK, Tse SM, Croteau-Chonka DC, Pino-Yanes M, Rosas-Salazar C, et al. Stress and Bronchodilator Response in Children with Asthma. Am J Respir Crit Care Med 2015; 192:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puranik S, Forno E, Bush A, Celedon JC. Predicting Severe Asthma Exacerbations in Children. Am J Respir Crit Care Med 2017; 195:854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, et al. Mind-body interactions in the regulation of airway inflammation in asthma: A PET study of acute and chronic stress. Brain Behav Immun 2016; 58:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberg SL, Miller GE, Brehm JM, Celedon JC. Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol 2014; 134:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. J Adolesc Health 2007; 41:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kewalramani A, Bollinger ME, Postolache TT. Asthma and Mood Disorders. Int J Child Health Hum Dev 2008; 1:115–23. [PMC free article] [PubMed] [Google Scholar]
- 126.Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. J Asthma 2005; 42:643–7. [DOI] [PubMed] [Google Scholar]
- 127.Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and anxiety and depression symptoms. Pediatrics 2006; 118:1042–51. [DOI] [PubMed] [Google Scholar]
- 128.Lacwik P, Szydłowska D, Kupczyk M, Pałczyński C, Kuna P. High levels of anxiety during the COVID-19 pandemic as a risk factor of clinical worsening in patients with severe asthma. J Allergy Clin Immunol Pract 2021; 9:1381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Griffiths D, Giancola LM, Welsh K, MacGlashing K, Thayer C, Gunnlaugsson S, et al. Asthma control and psychological health in pediatric severe asthma. Pediatr Pulmonol 2021; 56:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luria CJ, Sitarik AR, Havstad S, Zoratti EM, Kim H, Wegienka GR, et al. Association between asthma symptom scores and perceived stress and trait anxiety in adolescents with asthma. Allergy Asthma Proc 2020; 41:210–7. [DOI] [PubMed] [Google Scholar]
- 131.Munoz FA, Benton LD, Kops SA, Kowalek KA, Seckeler MD. Greater length of stay and hospital charges for severe asthma in children with depression or anxiety. Pediatr Pulmonol 2020; 55:2908–12. [DOI] [PubMed] [Google Scholar]
- 132.Bardach NS, Neel C, Kleinman LC, McCulloch CE, Thombley R, Zima BT, et al. Depression, Anxiety, and Emergency Department Use for Asthma. Pediatrics 2019; 144. [DOI] [PubMed] [Google Scholar]
- 133.Morillo-Vanegas D, Sanchez-Salcedo P, Sebastián Ariño AF. Relationship between pediatric asthma and psychosocial status of caregivers. Respir Med 2020; 174:106187. [DOI] [PubMed] [Google Scholar]
- 134.Kopel LS, Phipatanakul W, Gaffin JM. Social disadvantage and asthma control in children. Paediatr Respir Rev 2014; 15:256–62; quiz 62–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med 2001; 155:347–53. [DOI] [PubMed] [Google Scholar]
- 136.Licari A, Ciprandi R, Marseglia G, Ciprandi G. Anxiety and depression in adolescents with asthma and in their parents: a study in clinical practice. Monaldi Arch Chest Dis 2019; 89. [DOI] [PubMed] [Google Scholar]
- 137.Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics 2004; 113:229–37. [DOI] [PubMed] [Google Scholar]
- 138.Goodwin RD, Castro M, Kovacs M. Major depression and allergy: does neuroticism explain the relationship? Psychosom Med 2006; 68:94–8. [DOI] [PubMed] [Google Scholar]
- 139.Sastre J, Crespo A, Fernandez-Sanchez A, Rial M, Plaza V. Anxiety, Depression, and Asthma Control: Changes After Standardized Treatment. J Allergy Clin Immunol Pract 2018; 6:1953–9. [DOI] [PubMed] [Google Scholar]
- 140.Chiang LC, Ma WF, Huang JL, Tseng LF, Hsueh KC. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. Int J Nurs Stud 2009; 46:1061–70. [DOI] [PubMed] [Google Scholar]
- 141.Pateraki E, Morris PG. Effectiveness of cognitive behavioural therapy in reducing anxiety in adults and children with asthma: A systematic review. J Asthma 2018; 55:532–54. [DOI] [PubMed] [Google Scholar]
- 142.Marriage D, Henderson J. Cognitive behaviour therapy for anxiety in children with asthma. Nurs Child Young People 2012; 24:30–4. [DOI] [PubMed] [Google Scholar]
- 143.Kew KM, Nashed M, Dulay V, Yorke J. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev 2016; 9:Cd011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown ES, Sayed N, Van Enkevort E, Kulikova A, Nakamura A, Khan DA, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Escitalopram in Patients with Asthma and Major Depressive Disorder. J Allergy Clin Immunol Pract 2018; 6:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics 2009; 123:797–803. [DOI] [PubMed] [Google Scholar]
- 146.Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, et al. Vitamin D status in the United States, 2011–2014. Am J Clin Nutr 2019; 110:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, et al. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J 2011; 37:1366–70. [DOI] [PubMed] [Google Scholar]
- 148.Cassim R, Russell MA, Lodge CJ, Lowe AJ, Koplin JJ, Dharmage SC. The role of circulating 25 hydroxyvitamin D in asthma: a systematic review. Allergy 2015; 70:339–54. [DOI] [PubMed] [Google Scholar]
- 149.Mohammadzadeh I, Darvish S, Qujeq D, Hajiahmadi M, Vaghari-Tabari M. Association of serum 25-OH vitamin D3 with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc 2020; 41:126–33. [DOI] [PubMed] [Google Scholar]
- 150.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med 2010; 181:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010; 126:52–8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012; 186:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han YY, Forno E, Alvarez M, Colon-Semidey A, Acosta-Perez E, Canino G, et al. Diet, Lung Function, and Asthma Exacerbations in Puerto Rican Children. Pediatr Allergy Immunol Pulmonol 2017; 30:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010; 125:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA Jr., Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017; 5:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016; 9:Cd011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Forno E, Bacharier LB, Phipatanakul W, Guilbert TW, Cabana MD, Ross K, et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. Jama 2020; 324:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. Jama 2014; 311:2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh M, Paul N, Singh S, Nayak GR. Asthma and Fungus: Role in Allergic Bronchopulmonary Aspergillosis (ABPA) and Other Conditions. Indian J Pediatr 2018; 85:899–904. [DOI] [PubMed] [Google Scholar]
- 160.Agarwal R Severe asthma with fungal sensitization. Curr Allergy Asthma Rep 2011; 11:403–13. [DOI] [PubMed] [Google Scholar]
- 161.Agarwal R, Sehgal IS, Dhooria S, Aggarwal AN. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med 2016; 10:1317–34. [DOI] [PubMed] [Google Scholar]
- 162.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43:850–73. [DOI] [PubMed] [Google Scholar]
- 163.Shah A, Kunal S. A review of 42 asthmatic children with allergic bronchopulmonary aspergillosis. Asia Pac Allergy 2017; 7:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shah A, Panjabi C. Allergic aspergillosis of the respiratory tract. Eur Respir Rev 2014; 23:8–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vicencio AG, Santiago MT, Tsirilakis K, Stone A, Worgall S, Foley EA, et al. Fungal sensitization in childhood persistent asthma is associated with disease severity. Pediatr Pulmonol 2014; 49:8–14. [DOI] [PubMed] [Google Scholar]
- 166.Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014; 2:703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stevens DA, Schwartz HJ, Lee JY, Moskovitz BL, Jerome DC, Catanzaro A, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342:756–62. [DOI] [PubMed] [Google Scholar]
- 168.Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: A synthesis review of published literature. Respir Med 2017; 122:33–42. [DOI] [PubMed] [Google Scholar]
- 169.Dhariwal J, Hearn AP, Kavanagh JE, d’Ancona G, Green L, Fernandes M, et al. Real-World Effectiveness of Anti-IL-5/5R Therapy in Severe Atopic Eosinophilic Asthma with Fungal Sensitization. J Allergy Clin Immunol Pract 2021; 9:2315–20.e1. [DOI] [PubMed] [Google Scholar]
- 170.Schleich F, Vaia ES, Pilette C, Vandenplas O, Halloy JL, Michils A, et al. Mepolizumab for allergic bronchopulmonary aspergillosis: Report of 20 cases from the Belgian Severe Asthma Registry and review of the literature. J Allergy Clin Immunol Pract 2020; 8:2412–3.e2. [DOI] [PubMed] [Google Scholar]
- 171.Terashima T, Shinozaki T, Iwami E, Nakajima T, Matsuzaki T. A case of allergic bronchopulmonary aspergillosis successfully treated with mepolizumab. BMC Pulm Med 2018; 18:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramonell RP, Lee FE, Swenson C, Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: A case series. J Allergy Clin Immunol Pract 2020; 8:742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Denning DW, O’Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 2009; 179:11–8. [DOI] [PubMed] [Google Scholar]
- 174.Agbetile J, Bourne M, Fairs A, Hargadon B, Desai D, Broad C, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol 2014; 134:33–9. [DOI] [PubMed] [Google Scholar]
- 175.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 176.Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu J, et al. Effect of active smoking on asthma symptoms, pulmonary function, and BHR in adolescents. Pediatr Pulmonol 2009; 44:954–61. [DOI] [PubMed] [Google Scholar]
- 177.Alanazi AMM, Alqahtani MM, Pavela G, Ford EW, Leventhal AM, Hendricks PS. Mental Health and the Association between Asthma and E-cigarette Use among Young Adults in The United States: A Mediation Analysis. Int J Environ Res Public Health 2020; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van De Ven MO, van Zundert RM, Engels RC. Effects of asthma on nicotine dependence development and smoking cessation attempts in adolescence. J Asthma 2013; 50:250–9. [DOI] [PubMed] [Google Scholar]
- 179.Murray AB, Morrison BJ. The effect of cigarette smoke from the mother on bronchial responsiveness and severity of symptoms in children with asthma. J Allergy Clin Immunol 1986; 77:575–81. [DOI] [PubMed] [Google Scholar]
- 180.Weitzman M, Gortmaker S, Walker DK, Sobol A. Maternal smoking and childhood asthma. Pediatrics 1990; 85:505–11. [PubMed] [Google Scholar]
- 181.Evans D, Levison MJ, Feldman CH, Clark NM, Wasilewski Y, Levin B, et al. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis 1987; 135:567–72. [DOI] [PubMed] [Google Scholar]
- 182.Martinez FD, Antognoni G, Macri F, Bonci E, Midulla F, De Castro G, et al. Parental smoking enhances bronchial responsiveness in nine-year-old children. Am Rev Respir Dis 1988; 138:518–23. [DOI] [PubMed] [Google Scholar]
- 183.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med 2018; 6:526–34. [DOI] [PubMed] [Google Scholar]
- 184.Pyle RC, Divekar R, May SM, Narla N, Pianosi PT, Hartz MF, et al. Asthma-associated comorbidities in children with and without secondhand smoke exposure. Ann Allergy Asthma Immunol 2015; 115:205–10. [DOI] [PubMed] [Google Scholar]
- 185.Murray AB, Morrison BJ. The decrease in severity of asthma in children of parents who smoke since the parents have been exposing them to less cigarette smoke. J Allergy Clin Immunol 1993; 91:102–10. [DOI] [PubMed] [Google Scholar]
- 186.Global Initiative for Asthma (GINA), 2021. http://www.ginasthma.org/: http://www.ginasthma.org/, 2021.
- 187.Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH. Smoking and asthma. J Am Board Fam Med 2011; 24:313–22. [DOI] [PubMed] [Google Scholar]
- 188.Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. Faseb j 2001; 15:1110–2. [PubMed] [Google Scholar]
- 189.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007; 175:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Price D, Popov TA, Bjermer L, Lu S, Petrovic R, Vandormael K, et al. Effect of montelukast for treatment of asthma in cigarette smokers. J Allergy Clin Immunol 2013; 131:763–71. [DOI] [PubMed] [Google Scholar]
- 191.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS One 2015; 10:e0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2021. https://goldcopd.org/, 2021.
- 193.Bodkhe S, Nikam M, Sherje AP, Khan T, Suvarna V, Patel K. Current insights on clinical efficacy of roflumilast for treatment of COPD, asthma and ACOS. Int Immunopharmacol 2020; 88:106906. [DOI] [PubMed] [Google Scholar]
- 194.Eliasson O, Scherzer HH, DeGraff AC, Jr. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol 1986; 77:87–94. [DOI] [PubMed] [Google Scholar]
- 195.Gibbs CJ, Coutts II Lock R, Finnegan OC, White RJ. Premenstrual exacerbation of asthma. Thorax 1984; 39:833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma 2002; 39:517–22. [DOI] [PubMed] [Google Scholar]
- 197.Rao CK, Moore CG, Bleecker E, Busse WW, Calhoun W, Castro M, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 2013; 143:984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brenner BE, Holmes TM, Mazal B, Camargo CA. Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax 2005; 60:806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zimmerman JL, Woodruff PG, Clark S, Camargo CA. Relation between phase of menstrual cycle and emergency department visits for acute asthma. Am J Respir Crit Care Med 2000; 162:512–5. [DOI] [PubMed] [Google Scholar]
- 200.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol 2018; 120:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Macsali F, Real FG, Omenaas ER, Bjorge L, Janson C, Franklin K, et al. Oral contraception, body mass index, and asthma: a cross-sectional Nordic-Baltic population survey. J Allergy Clin Immunol 2009; 123:391–7. [DOI] [PubMed] [Google Scholar]
- 202.Nwaru BI, Sheikh A. Hormonal contraceptives and asthma in women of reproductive age: analysis of data from serial national Scottish Health Surveys. J R Soc Med 2015; 108:358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81:509–17. [PubMed] [Google Scholar]
- 204.Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol 2003; 112:283–8. [DOI] [PubMed] [Google Scholar]
- 205.Murphy VE, Gibson PG, Talbot PI, Kessell CG, Clifton VL. Asthma self-management skills and the use of asthma education during pregnancy. Eur Respir J 2005; 26:435–41. [DOI] [PubMed] [Google Scholar]
- 206.Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol 2010; 115:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu TD, Brigham EP, Peng R, Koehler K, Rand C, Matsui EC, et al. Overweight/obesity enhances associations between secondhand smoke exposure and asthma morbidity in children. J Allergy Clin Immunol Pract 2018; 6:2157–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


