Abstract
Introduction: The clinical effect of low-level laser therapy (LLLT) on canine wounds is still under debate. The aim of this pilot study was to evaluate the potential influence of LLLT on the bacterial loads of wounds, using two different energy densities or doses of laser light as an adjuvant therapy for traumatic contaminated wound management.
Methods: A prospective, randomized, blinded, placebo-controlled pilot clinical trial was used to evaluate the effect of two different doses of LLLT as an adjuvant treatment of contaminated traumatic wounds on the bacterial load and wound scoring in dogs. Fourteen dogs with traumatic bites or laceration wounds were randomly assigned to one of the three groups. Animals in groups A and B received a dose of LLLT of 6 and 2 J/cm2 respectively. Four wavelengths were used simultaneously: 660 nm, 800 nm, 905 nm, and 970 nm. Animals in group C received placebo LLLT. Bacterial burden and clinical wound scores were evaluated. Results: A statistically significant reduction in the average count of colony forming units was observed in group B (2 J/cm2) when compared to placebo group C. Group B also showed improved wound scores. No clinically adverse effects were observed in the patients treated with LLLT.
Conclusion: LLLT, with the parameters used in this pilot trial, decreased bacterial loads of contaminated wounds in dogs and improved wound scores, especially when using a dose of 2 J/ cm2. This is the first time the effect of LLLT on bacterial load has been investigated in a clinical setting using traumatic wounds in canine patients.
Keywords: Laser therapy, Wounds, Bacterial count/bacterial load
Introduction
Low-level laser therapy (LLLT) is a form of photobiomodulation (PBM) that results in a biological effect from a light source. It is a non-invasive physical modality, both versatile and safe, and is becoming a popular and valuable tool for clinicians.1,2 Nevertheless, questions concerning some of the biological effects such as its clinical impact on wounds, especially contaminated and infected wounds, remain unanswered.
The mechanism of action of LLLT is based on chromophores, i.e., molecules, such as water, hemoglobin, or cytochrome-C oxidase, which are stimulated when they absorb the energy emitted by the laser in the form of photons.3-5 When this happens, local blood flow, oxygenation, and metabolism are enhanced, thus increasing the efficiency of the mitochondrial respiratory chain, ATP production, and the oxidative burst of neutrophils and macrophages.6 Intracellular signaling and secondary reactions eventually translate as DNA and RNA synthesis, cell multiplication, release of growth factors and neurotransmitters, and increased collagen production.7 This can be summarized as an improvement of tissue metabolism and functionality.8
The most documented effects of LLLT are the ones on wound healing, inflammation, and pain. To date, most research has been performed on experimental animals, but a growing body of evidence includes clinical studies in humans and a few studies in domestic animal species.9-12
Bacterial proliferation in wounds can negatively affect healing and has a significant impact on treatment costs.13,14 This, together with growing concerns about antibiotic resistance, explains why there is an urgent and increasing need for alternative treatments. Authors who have focused their research on the effect of LLLT on bacteria have performed either in vitro experiments15,16or in vivo studies using experimental animals.17-19 Results have varied depending on the methodology, especially the irradiation and treatment parameters such as dose (J/cm2), wavelength (nm), and power density (W/cm2).20 To the authors’ knowledge, no clinical trial using laser therapy for canine patients has been published that concerns acute traumatic, contaminated/infected wounds in particular. The aim of this pilot study was to evaluate the potential influence of LLLT on the bacterial loads of wounds, using two different doses or energy densities of laser light as an adjuvant therapy for contaminated wound management.
Materials and Methods
Study Design
A prospective, randomized, blinded, and placebo-controlled pilot clinical trial was performed in three parallel groups. This study received the approval of the Ethics and Animal Experimentation Committee of the two universities, located in Madrid, Spain. Patient owners were given information about the protocol to be followed and they signed an informed consent form to include their dogs in the study.
Simple randomization was used to allocate each patient to one of the groups (A, B or C) using the statistical software package Stata 13.0 (Stata Corp., College Station, TX, USA). Patient owners, the colony forming units (CFUs) evaluator, and the data analyst were blinded to the treatment group. The only person who knew which group patients belonged to was the clinician who applied the laser treatment.
Sample Population
Patients were included in the study after meeting inclusion criteria. Dogs presenting with traumatic wounds with signs of contamination or infection and no previous treatment were considered. According to the Center for Disease Control and Prevention, contaminated wounds include open, fresh, accidental wounds as well as incisions with acute non-purulent inflammation21; patients with clinical signs of infection or some degree of devitalized tissue were also included. The initial inclusion of the patients was reassessed 24 hours later, after laboratory confirmation of wound contamination.
Laser Treatment
The patients received their assigned treatment on days 0 and 1, according to the experimental group they had been allocated to. Two samples were obtained from each patient for their microbiological analysis: the first sample was taken on day 0, before the first laser treatment, and the second sample on day 2 (Table 1).
Table 1. Study Design: Timeline of Interventions .
| ↓ ↓ ↓ ↓ ↓ ↓ |
⟶⟶⟶⟶⟶⟶⟶⟶ | ||
| Day 0 | Day 1 | Day 2 | |
| Microbiology sample named “before treatment”(BT) | Microbiology sample named “after treatment” (AT) | ||
| LLLT | LLLT | LLLT | |
| Wound management | Wound management | Wound management | |
| Antibiotic | |||
Groups A and B received an energy dose of 6 J/cm2 and 2 J/cm2 respectively. Group C received the placebo laser treatment (Table 2). The therapeutic laser was a class IV type, a K-laser® Cube 4 (Eltech K-Laser, Treviso, Italy), which was tested for optical output before and after the end of the trial. This device allowed us to use different frequencies, as well as continuous wave (CW), over the course of the treatment. In particular, an initial CW phase was followed by three pulsed light phases at 500 Hz, 5000 Hz and 20 000 Hz. The device delivered 25% of the energy using CW, and the remainder was equally divided between the pulsed phases. In the pulsed mode, the device had a 50% duty cycle, which meant that while the average power was kept at 2 W, the peak power was 4 W. Treatments were performed in non-contact mode, and the diameter of the laser beam over the patient was adjusted to 10 cm2. Therefore, the average power density was 0.2 W/cm2. Four wavelengths were used simultaneously: 660 nm, 800 nm, 905 nm, and 970 nm. Treatment time was adapted to the size of the treatment area and to the applied dose. The device also had a placebo mode that produced a 4 mW red light and a beeping sound similar to the treatment mode. This placebo mode was used for the control group.
Table 2. Treatment Parameters for Each Group .
| Group | Dose | Wavelength | Frequency | Power |
| A | 6 J/cm2 | 660 nm 800 nm 905 nm 970 nm |
CW 50 Hz 5000 Hz 20000 Hz |
2 W Pa 4 W Pp |
| B | 2 J/cm2 | |||
| C | Placebo: 5 mW, 660 nm |
CW, continuous wave; Pa, average power; Pp, peak power.
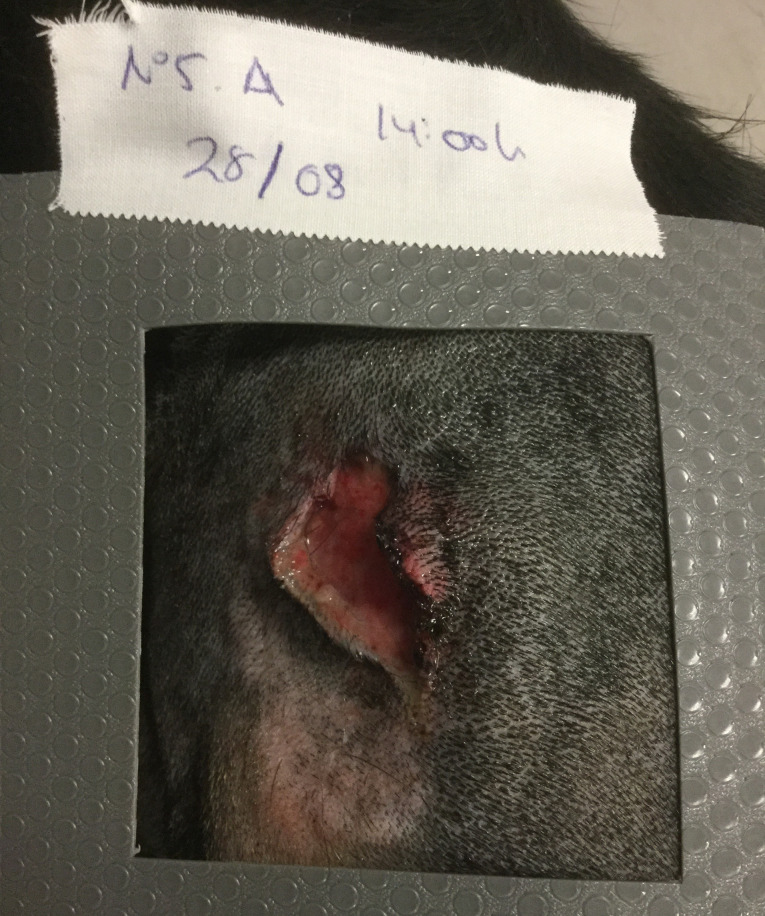
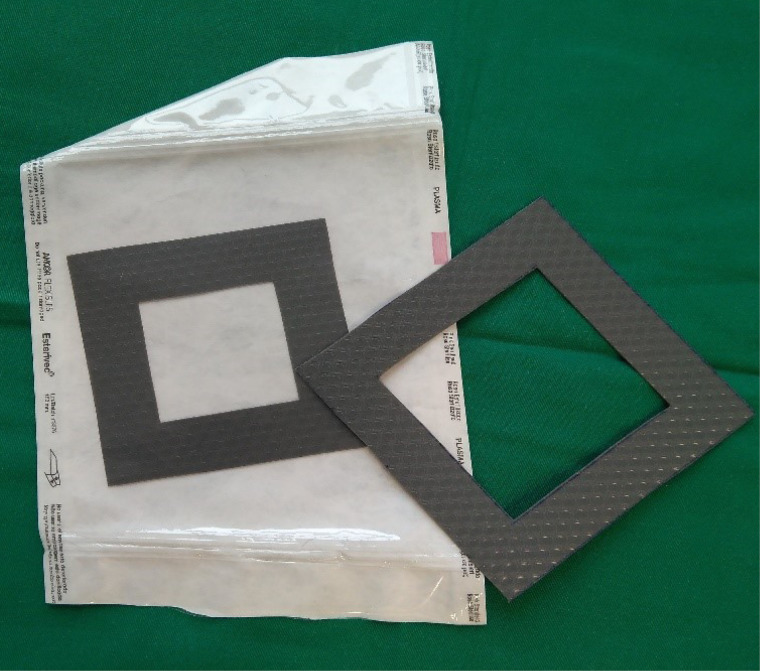
The treatment area for each wound included the wound itself plus a margin of 2 to 4 cm of healthy tissue. The area to be treated and sampled was delimited using a series of pre-cut sterile silicone templates (Figures 1 and 2) of 25, 50, and 100 cm2. This allowed us to calculate the bacterial load for each patient in a uniform way.
Figure 1.
A sample of the pre-cut silicone templates of different sizes used in the study.
Figure 2.
Silicone template over a traumatic wound, limiting the area to be treated and subsequently sampled for bacteriology.
Standard Management
Once irradiated, wounds were lavaged with Lactated Ringer’s solution, using 50 cc for each cm2 of the wound, with an average pressure of 300 mm Hg. Pressure control was achieved with a pressure infuser bag. Both the laser treatment and wound management were repeated on day 1 of the experiment.
For the more severe or complicated wounds, the patients were sedated on day 0 for treatment and sampling whenever it was clinically indicated.
All patients received standardized medical treatment after laser therapy. This included a subcutaneous injection of a third-generation cephalosporin dose (cefovecin, 8 mg/kg of body weight), so the only treatment difference among groups was the LLLT dose. Each dog wore an E-collar for at least the first 72 hours to prevent wound licking.
The patients were rechecked on a daily basis for the first 72 hours, and subsequent visits were planned according to each patient’s needs until wound healing was complete. All data concerning clinical progression were recorded.
Microbiology
The samples were collected from the wounds using a sterile cotton swab, previously immersed in 1 cc of phosphate buffered saline. Once in the laboratory, and starting with 0.1 cc of the original sample, three progressive dilutions were performed, each ten times more diluted than the previous dilution. Then, 0.1 cc of each of the four dilutions was cultured using both Columbia blood agar and McConkey agar culture plates, which were incubated in aerobic conditions for 24 hours at 37ºC and read after incubation. Plates containing between 30 and 300 CFUs were selected as reading plates. The bacterial load (CFUs/cm2) in each wound of each patient was calculated from these reading plates.
Each morphologically distinct strain was isolated and stored at -40ºC. The identification of bacterial species was performed at the Centre for Veterinary Sanitary Surveillance of the Complutense University of Madrid (VISAVET), using mass spectrophotometry, with Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF).
Data Collection
The Neperian logarithm of CFUs (LNCFUs) after treatment (AT) in the Columbia blood agar and McConkey agar culture plates was selected as the outcome variable. Independent variables were classified into clinical variables (breed, age, reproductive status, and body weight), wound-specific variables (number of wounds, origin, and area), treatment-specific variables (treatment area, experimental group, additional treatments, and time between first and second sampling) and microbiological variables (LNCFUs before treatment).
Wound Scoring
The pictures of the wounds were taken on day 0, day 1 and day 2 before laser therapy. Wound scoring was performed in a blinded way by two evaluators with more than15 years of experience in soft tissue surgery. The scoring system used was a modification from the scale proposed by Falanga et al.22 In this way, scoring could range from 2 to 12 since at least a positive culture was necessary on day 0 for the patient to be included in the study (Table 3).
Table 3. Wound scoring scale .
| Clinical Parameter | 0 | 1 | 2 |
| Exudate amount | Severe or purulent type | Moderate | None/mild |
| Edema or swelling | Severe | Moderate | None/mild |
| Peri-wound dermatitis | Severe | Moderate | None or mild/minimal |
| Hematoma | Severe | Moderate | None/minimal |
| Necrotic or potentially non-viable tissue | > 25% of wound surface | 0-25% | None |
| Bacterial culture | Positive | Negative |
Statistical Analysis
Continuous variables are presented as means and standard deviations, or medians and interquartile ranges. Categorical variables are expressed as frequencies and percentages.
To analyze the influence of different variables in the LNCFUs AT, a multivariate linear regression model was created. Variables with P < 0.100 were considered clinically relevant and included in the multivariate linear regression analysis. The final model was built with a stepwise forward selection and backward elimination technique. The significance levels for forward selection were P < 0.050 and for backward elimination were P < 0.100.
The results are shown as the ratio of estimated means for each variable. In the quantitative variables, this ratio expresses the relative increase of the mean per each one-unit increase in the independent variable. In the qualitative variables (groups), the ratio expresses the average increase of patients treated in group A or B, while using group C as the reference group. The ratio is obtained by exponentiation to base e of the coefficient of the linear regression model (β).
All tests were two-sided, and differences were considered statistically significant at P < 0.025. Bonferroni adjustments were used to correct for multiple comparisons. Statistical analyses were performed using Stata software version 13.0 (Stata Corp) and the Statistical Package for the Social Sciences (SPSS 15.0, IBM, NY, USA).
For wound scoring, inter-rater agreement was evaluated using Cronbach’s alpha coefficient. Total scoring for each wound each day was the average of both evaluators’ scoring. Total scores for days 0 and 2 were compared using a t-test for independent measures for groups A, B and C.
Results
Fourteen canine patients initially met the inclusion criteria. None of these animals had to be excluded since bacterial contamination was confirmed in all cases. Four patients were randomly assigned to group A, while groups B and C had five patients each. Group A included two Jack Russell Terriers (a male and a female, both intact), a Burgos Pointer (spayed female) and a Bodeguero Andaluz (intact male). The median age in group A was 5 years (1.2–7.5 years) and the median weight was 9.5 kg (10–15 kg). Group B included a Greyhound (spayed female), a Jagdterrier (spayed female) and three mixed-breed dogs (two spayed females and one intact male) with a median age of 8 years (4–9 years) and a median weight of 15 kg (8–23 kg). Group C included two Greyhounds (both spayed females), a German Shepherd (neutered male), an Ibizan Hound (neutered male) and a mixed-breed spayed female with a median age of 7 years (5–10 years) and a median weight of 23.6 kg (20–32 kg). Three patients from group A had bite wounds. The fourth patient presented with a traumatic cut. Groups B and C included three patients each with bite wounds and two with traumatic cuts. Each patient had between one and four wounds (average 1.5 wounds), but only one wound from each patient was included in the study. In group A, laser treatment and sampling were done over 25 cm2 for three patients and over 100 cm2 for the fourth dog. In group B, three patients were treated and sampled from 25 cm2, and two patients from 50 cm2. Group C included three patients who were treated and sampled from 25 cm2 and two patients from 100 cm2.
Sedation was performed in ten patients on day 0 according to their needs for appropriate wound management. None of the patients had to be sedated again or undergo further surgical management for the treated wound, and no dog needed additional antibiotic therapy during the days of the trial.
Results from bacterial identification are shown in Table 4. Among the 77 isolated bacterial strains, 8 could not be identified due to lack of survival related to problems during storing, defrosting and /or sample processing.
Table 4. Microbiology Results: Bacterial Identification .
| No. bacteria | Patient | Culture | Bacteria | Gram |
|---|---|---|---|---|
| 1 | 1 | BT | Staphylococcus pseudintermedius | + |
| 2 | 1 | BT | Aerococcus viridans | + |
| 3 | 1 | BT | Neisseria zoodegmatis | - |
| 4 | 1 | AT | Bacillus licheniformis | + |
| 5 | 1 | AT | Escherichia coli | - |
| 6 | 1 | AT | Escherichia coli | - |
| 7 | 1 | AT | Staphylococcus pseudintermedius | + |
| 8 | 1 | AT | Staphylococcus pseudintermedius | + |
| 9 | 2 | BT | Aerococcus viridans | + |
| 10 | 2 | BT | Neisseria zoodegmatis | - |
| 11 | 2 | BT | Neisseria weaveri | - |
| 12 | 2 | AT | Staphylococcus pseudintermedius | + |
| 13 | 3 | BT | Rothia nasimurium | + |
| 14 | 3 | BT | Neisseria zoodegmatis | - |
| 15 | 3 | BT | Actinobacillus pleuropneumoniae | - |
| 16 | 3 | AT | Staphylococcus spp. | + |
| 17 | 3 | AT | -- | -- |
| 18 | 3 | AT | Staphylococcus spp. | + |
| 19 | 4 | BT | Streptococcus sp. | + |
| 20 | 4 | BT | Staphylococcus aureus | + |
| 21 | 4 | AT | Streptococcus sp. | + |
| 22 | 4 | AT | Staphylococcus aureus | + |
| 23 | 4 | AT | Staphylococcus hominis | + |
| 24 | 5 | BT | Micrococcus spp. | + |
| 25 | 5 | BT | Streptococcus sp. | + |
| 26 | 5 | BT | Rothia nasimurium | + |
| 27 | 5 | AT | -- | -- |
| 28 | 5 | AT | -- | -- |
| 29 | 5 | AT | Acinetobacter baumannii | + |
| 30 | 5 | AT | Kocuria rhizophila | + |
| 31 | 5 | AT | Micrococcus luteus | + |
| 32 | 6 | BT | Staphylococcus aureus | + |
| 33 | 6 | BT | Staphylococcus pseudintermedius | + |
| 34 | 6 | AT | Staphylococcus pseudintermedius | + |
| 35 | 6 | AT | Staphylococcus spp. | + |
| 36 | 7 | BT | Pasteurella multocida | - |
| 37 | 7 | BT | Actinobacillus pleuropneumoniae | - |
| 38 | 7 | BT | Pasteurella canis | - |
| 39 | 7 | AT | Neisseria flavescens | - |
| 40 | 7 | AT | -- | -- |
| 41 | 8 | BT | Neisseria canis | - |
| 42 | 8 | BT | Neisseria flavescens | - |
| 43 | 8 | AT | Staphylococcus warneri | + |
| 44 | 8 | AT | Staphylococcus pseudintermedius | + |
| 45 | 9 | BT | Staphylococcus epidermidis | + |
| 46 | 9 | BT | Micrococcus spp. | + |
| 47 | 9 | BT | Acinetobacter spp. | + |
| 48 | 9 | BT | Neisseria zoodegmatis | - |
| 49 | 9 | AT | Enterococcus faecium | + |
| 50 | 10 | BT | Neisseria weaveri | - |
| 51 | 10 | BT | Bergeyella zoohelcum | - |
| 52 | 10 | BT | Streptococcus hyointestinalis | + |
| 53 | 10 | BT | -- | -- |
| 54 | 11 | BT | Acinetobacter spp. | + |
| 55 | 11 | BT | Staphylococcus spp. | + |
| 56 | 11 | BT | Neisseria zoodegmatis | - |
| 57 | 11 | AT | Bacillus sp. | + |
| 58 | 11 | AT | Staphylococcus delphini | + |
| 59 | 11 | AT | Staphylococcus epidermidis | + |
| 60 | 11 | AT | Streptococcus salivarius | + |
| 61 | 12 | BT | Staphylococcus hominis | + |
| 62 | 12 | BT | Corynebacterium afermentans | + |
| 63 | 12 | BT | Mannheimia haemolytica | - |
| 64 | 12 | BT | Corynebacterium tuberculostearicum | + |
| 65 | 12 | AT | Staphylococcus hominis | + |
| 66 | 12 | AT | Arthrobacter sp. | + |
| 67 | 12 | AT | -- | -- |
| 68 | 13 | BT | -- | -- |
| 69 | 13 | BT | Acinetobacter spp. | + |
| 70 | 13 | BT | -- | -- |
| 71 | 13 | AT | Staphylococcus spp | + |
| 72 | 14 | BT | Bergeyella zoohelcum | - |
| 73 | 14 | BT | Acinetobacter spp | + |
| 74 | 14 | BT | Neisseria zoodegmatis | - |
| 75 | 14 | AT | Staphylococcus spp | + |
| 76 | 14 | AT | Acinetobacter spp | + |
| 77 | 14 | AT | Acinetobacter spp. | + |
BT, before treatment. AT, after treatment.
Statistical analysis did not reveal any influence of the clinical variables or wound-specific variables over the number of CFUs AT in Columbia blood agar. Conversely, we observed a relative decrease of 79% (mean ratio 0.21) and 93% (mean ratio 0.07) was observed in the average growth of LNCFUs in Columbia blood agar from day 0 to day 2 for groups A and B respectively. The analysis was adjusted by the time between samples and by the number of LNCFUs in Columbia blood agar. This change was statistically significant in group B (P< 0.050). The mean of LNCFUs after treatment increased (P = 0.018) 1.47 times the mean of the initial number of LNCFUs in Columbia blood agar, adjusted by the time between the samples and the group. The mean of LNCFUs after treatment increased (P < 0.001) 3.76 times per additional hour between the samples, adjusted by the LNCFUs before treatment and the group. This linear regression model is shown in Table 5. No statistically significant results were retrieved from the McConkey agar plates (Table 6).
Table 5. Linear Regression Model of LNCFUs AT in Columbia Blood Agar Plates in Relation to LNCFUs BT, Time Between Samples, and Treatment Groups .
| Columbia Blood Agar (LNCFUs AT) | Coefficient (β) | Mean Ratio | 95% CI Lower | 95% CI Upper | P Value | |
|---|---|---|---|---|---|---|
| Columbia Blood Agar (LNCFUs BT) | 0.38 | 1.47 | 0.07 | 0.70 | 0.018* | |
| Time between samples | 1.33 | 3.76 | 0.80 | 1.85 | < 0.001* | |
| Laser | Group C | 1 | ||||
| Group A | -1.55 | 0.21 | -3.91 | 0.82 | 0.210 | |
| Group B | -2.73 | 0.07 | -4.36 | -1.09 | 0.001* |
LNCFUs AT, Neperian logarithm of colony-forming units after treatment; LNCFUs BT, Neperian logarithm of colony-forming units before treatment.
*Statistically significant
Table 6. Linear Regression Model of LNCFUs AT in McConkey Agar Plates in Relation to LNCFUs BT, Time Between Samples, and Treatment Groups .
| McConkey Agar (LNCFUs AT) | Coefficient (β) | Mean Ratio | 95% CI Lower | 95% CI Upper | P Value | |
|---|---|---|---|---|---|---|
| McConkey Agar (LNCFUs BT) | -0.003 | 1.00 | -0.051 | 0.045 | 0.886 | |
| Time between samples | -0.003 | 1.00 | -0.025 | 0.019 | 0.780 | |
| Laser | Group C | 1 | ||||
| Group A | -0.003 | 1.00 | -0.092 | 0.086 | 0.939 | |
| Group B | -0.014 | 0.99 | -0.102 | 0.074 | 0.731 |
LNCFUs AT, Neperian logarithm of colony-forming units after treatment; LNCFUs BT, Neperian logarithm of colony-forming units before treatment.
No adverse effects were observed in the patients treated with LLLT. Cronbach’s alpha coefficient between the two evaluators was 0.793. There were no differences in wound scores on day 0 between the groups. All patients improved their wound score from day 0 to day 2. In group A (6 J/cm2), the difference between means from day 0 to day 2 was 1.63 (95% CI: 0.06-3.31; P = 0.056). In group B (2 J/cm2), the difference between means from day 0 to day 2 was 2.9 (95% CI: 1.56-4.2; P = 0.002). In group C (placebo), the difference between means from day 0 to day 2 was 2.00 (CI 95%, 1.39-2.61; P < 0.001). When the results on day 2 were compared for different groups, the difference between means between groups B and C was 1.30 (95% CI: 0.67-1.93, P = 0.001); the difference between means between groups A and C was 1.00 (95% CI: 1.29-1.33, P = 0.853), and the difference between means between groups A and B was 1.20 (95% CI: 2.44-0.04, P = 0.056).
Discussion
In the canine species, the use of PBM has been described in individual clinical cases23 and in studies with clean surgical wounds,12,24-26 but this is the first pilot clinical trial in canine patients with traumatic wounds. To the best of the authors’ knowledge, it is also the first one with canine patients, in which PBM with infrared light is used as an adjuvant therapy to fight tissue infection, an effect that has been described in experimental studies in rats27-31and rabbits.32 More recently, a different type of PBM, using visible fluorescent light and a transducer gel was used in the treatment of canine pyoderma.33 In human patients, some studies report the use of LLLT to treat infected wounds in skin and mucosas.34 Investigating new tools to fight infection is a priority nowadays since antibiotic resistance has become a global public health threat with a significant impact on mortality, morbidity and the financial costs of medical care.13,14
The main result from our pilot clinical trial is that LLLT, or PBM, decreased the average number of bacteria in the post-treatment blood agar plates when compared to the placebo group. Although there is a difference between the bacterial load and tissue infection, a higher bacterial load, especially from some pathogenic bacteria, can contribute to the development of tissue infection and interferes with wound healing.35,36 The bacterial load was used as the main diagnostic criteria to objectively diagnose infection, although these criteria were later modified.35 The high positivity of bacteriology and the presence of polymicrobial flora were in accordance with what has been described in the literature, especially concerning bite wounds.36,37
The effect of PBM on infected tissue can be explained by the effect on bacteria themselves, i.e., their survival and multiplication, by the effect in the host’s immune response, or by a combination of both. Previous studies have contributed to answers to the question of what type of effect the treatment has on bacteria using both in vitro and in vivo research studies, but the lack of standardized parameters over a variety of microorganisms has produced uneven conclusions.
Some in vitro studies show an antibacterial effect,15,18,38and other studies describe no effect or even an increase in bacterial multiplication.39-41 One of the direct antibacterial mechanisms that has been proposed is based on the action of the light on the pathogen’s endogenous porphyrins.6,42 These porphyrins are thought to be stimulated by laser light, setting off a release of free radicals, which then damages cytoplasmic protein membranes and DNA. This bactericidal or bacteriostatic effect occurs when cellular damage overcomes repair mechanisms and microbial metabolism is inhibited. Some authors describe differences in efficacy when various pathogens are irradiated.15,43,44 Gram-negative bacteria seem to be more vulnerable. This could be due to differences in the peptidoglycan wall. This wall is thicker in gram-positive bacteria and therefore could account for more limited photon penetration.15,41 In the present study, however, McConkey agar plates, which favor Gram-negative bacteria, did not show a decreased bacteria load in response to LLLT, while Columbia blood agar plates did.
Our results are in accordance with those of other authors that have described an inhibition of bacterial growth on rat models of wounds,45 burns19,27 and osteomyelitis.28 In addition to the potential direct effect on bacterial survival,39,41,43 several phenomena in the host’s response are modulated by laser therapy and may contribute to the indirect antimicrobial effect, such as the increase in local blood flow and oxygenation.46-51 This facilitates the arrival of white blood cells and an improvement in the patients’ immune responses.52,53
The difference between groups in the present study was the randomly assigned laser treatment, but otherwise patient management was similar. Wound lavage and management were standardized for all patients as suggested by Balsa and Culp.54Cefovecin was selected as the antibiotic treatment because of its broad spectrum and its long-lasting and constant therapeutic levels in tissues after a single dose.55 This eliminated potential fluctuations in antibiotic concentration below the minimum inhibitory concentration values for the pathogens, as well as lack of treatment compliance by patient caretakers.56 The heterogeneity of laser technical and treatment parameters used in LLLT studies is a major challenge when comparing research results. This pilot study was performed in such a way that, dose (0, 2 or 6 J/cm2) was the only variable between the experimental groups. These doses (2-6 J/cm2) are similar to what is done in clinical practice with acute wounds57and within the range of other studies for wound management.24,26,58
In this pilot clinical trial, the results favored the use of a lower dose (2 J/cm2). This is in accordance with the recommended dosefor acute wounds in clinical practice (1–4 J/cm2), although both our 2 J/cm2and our 6 J/cm2doses produced a decrease in CFU counts. An inhibition of Staphylococcus aureus growth with a dose of 5 J/cm2was previously reported by Gomes et al30 and Santos et al29and also with lower dosages (1.2 and 2.4 J/cm2) on second-degree skin burns in rats according to Bayat et al.27 An inhibition of bacterial counts after LLLT was also described in an osteomyelitis model in rats by Kaya et al,28 who used higher dosages in their trial (7.64–22.9 J/cm2 vs. 2–6 J/cm2) although with lower power density (0.127 W/cm2 vs. 0.2 W/cm2).38 On the contrary, Araujo et al did not observe any antibacterial effect on a model of septic arthritis in rats, but it should be noted that although a similar dose was used (2 J/cm2), the intra-articular penetration of the 660-nm wavelength that was used was very limited.59 Vasheghani et al did not observe an antimicrobial effect on third-degree burns in rats either, but in their case, a much lower dose (0.396 J/cm2) was used.60The use of doses above 10 J/cm2 for wounds remains controversial. Some authors report a lack of stimulatory effect when higher doses are used, or even an inhibitory effect for in vitro cell metabolism,61,62 but others have reported positive effects in vivo with 30 J/cm2.63 In clinical practice, doses in the 10-30 J/cm2 range are sometimes used, especially in chronic wounds.
The selected power was within the usual range for acute wound treatment with class IV lasers.12,57Power density was kept to an average of 0.2 W/cm2 and this is within the 0.1–0.5 W/cm2 of the recommended range set for wounds by the World Association for Laser Therapy. This value was also congruent with that used by De Sousa et al in their 2016 study.38 While a certain amount of power density is needed to achieve a biological effect of PBM, an excessive power density can cause discomfort and tissue damage through unwanted photothermal effects. While the power of a laser can be focused on a power density that can kill bacteria due to a thermal effect, the aim of LLLT, as a form of PBM, is to modulate the metabolism of the tissue to enhance the process of healing. This has to be achieved at physiologic temperatures, which are safe and comfortable for the patient. Wavelength also has a direct influence on microbial chromophores and has been proposed as a potential mechanism for the antibacterial effect of light. Although blue light (380–475 nm) lacks some of the biological effects of red and infrared light on inflammation and wound healing, its antimicrobial properties have often been reported.38,64,65 These antimicrobial effects are still under discussion in the case of the red and infrared light used in PBM. We decided to combine all four wavelengths available in this device (660, 800, 905, and 970 nm) to reproduce what is usually done in practice when such combinations are available and to maximise the spectrum of cellular targets, similar to that done by Renwick et al.2
Our device allowed a pulsing range from 1 to 20 000 Hz, and we used a combination of CW, low, medium and high frequencies. The use of CW has been most commonly reported in the literature,28,41,66 but other authors have also used pulsed light up to 20 000 Hz.39,67 The significance of pulsing is still controversial, although it does seem to be of clinical benefit.68
Our results with wound scores seemed to be consistent with a progression in time and with the microbiological changes between the groups. Improved wound healing was also described in other clinical studies. Wardlaw et al. reported improved wound healing in canine patients treated with PBM after hemilaminectomy surgery12 using 8 J/cm2 and a combination of 850 nm and 670 nm. Perego et al,25 using 808 nm, described visible clinical improvement of post-operative healing of elective ovariectomy surgical wounds; although the global difference was not statistically significant, there was a statistically significant reduction in the exudate in the areas that had been treated. On the other hand, two other studies using dogs subjected to experimental surgical wounds found no difference in wound healing. Their parameters were very different though; while Kurach et al24used a class II laser at a wavelength of 635 nm, Gammel et al26used a class IV laser set at 2-3 W, a 980-nm wavelength and a dose of 5 J/cm2.
The present trial included a limited number of dogs with a variety of wound types, and this could limit and challenge the interpretation of our results. Further, the different etiopathogeneses of the investigated wounds could have influenced a different clinical progression, even under the same therapeutic protocol. Breed, sex, reproductive status, or body weight did not seem to influence the results, and the difference in bacterial growth achieved statistical significance.
Although the results of the clinical progression scores of the wounds were consistent with progression in time and with the reduction of the bacterial load, these results are limited by the low number of cases, the short time lapse during which wounds were scored and by the scoring method itself.
In the future, the inclusion of one or more groups in which antibiotic treatment would not be provided, anaerobic cultures and a longer period of scoring and microbiological sampling could retrieve more results and help to interpret our findings.
In summary, LLLT as an adjunctive treatment was able to decrease the bacterial burden in the wounds in both treatment groups; the effect was more significant with a dose of 2 J/cm2. The results from this pilot clinical trial are specific to the described treatment parameters and methodology, but they reinforce the growing scientific background supporting the clinical use of LLLT. Furthermore, they constitute a promising step forward in finding new strategies to treat infected wounds.
Ethical Considerations
This study received the approval of the Ethics and Animal Experimentation Committee of both Complutense University and Alfonso X el Sabio University, Madrid, Spain.
Conflict of Interests
MSR has received honoraria for K-Laser and AVANVET-sponsored speaking engagements. None of these circumstances has had an inappropriate influence on the content of this paper. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of this paper. The therapeutic laser used in this study was loaned to us by AVANVET, the Spanish distributor of K-Laser, who did not play any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The laser therapy device used in this trial was kindly lent to us by AVANVET. In addition, the Alfonso X El Sabio Foundation collaborated with the funding of the laboratory supplies that were needed to carry out this clinical trial.
Please cite this article as follows: Rico-Holgado S, Ortiz-Díez G, Martín-Espada MC, Fernández-Pérez C, Baquero-Artigao MR, Redondo MS. Effect of low-level laser therapy on bacterial counts of contaminated traumatic wounds in dogs. J Lasers Med Sci. 2021;12:e78. doi:10.34172/jlms.2021.78.
References
- 1.Pryor B, Millis DL. Therapeutic laser in veterinary medicine. Vet Clin North Am Small Anim Pract. 2015;45(1):45–56. doi: 10.1016/j.cvsm.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Renwick SM, Renwick AI, Brodbelt DC, Ferguson J, Abreu H. Influence of class IV laser therapy on the outcomes of tibial plateau leveling osteotomy in dogs. Vet Surg. 2018;47(4):507–515. doi: 10.1111/vsu.12794. [DOI] [PubMed] [Google Scholar]
- 3.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 4.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–58. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Lin Y, Gao S, Shen J. Study on mechanism of release oxygen by photo-excited hemoglobin in low-level laser therapy. Lasers Med Sci. 2018;33(1):135–139. doi: 10.1007/s10103-017-2363-y. [DOI] [PubMed] [Google Scholar]
- 6.Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg. 2005;23(1):3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- 7.Andrade Fdo S, Clark RM, Ferreira ML. Effects of low-level laser therapy on wound healing. Rev Col Bras Cir. 2014;41(2):129–33. doi: 10.1590/s0100-69912014000200010. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann N Y Acad Sci. 2005;1056:486–93. doi: 10.1196/annals.1352.040. [DOI] [PubMed] [Google Scholar]
- 9.Toida M, Watanabe F, Goto K, Shibata T. Usefulness of low-level laser for control of painful stomatitis in patients with hand-foot-and-mouth disease. J Clin Laser Med Surg. 2003;21(6):363–7. doi: 10.1089/104454703322650176. [DOI] [PubMed] [Google Scholar]
- 10.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;22(4):323–9. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 11.Jann HW, Bartels K, Ritchey J, Payton ME, Bennett J. Equine wound healing: influence of low level laser therapy on an equine metacarpal wound healing model. Photonics Lasers Med. 2012;1(2):117–122. doi: 10.1515/plm-2012-0004. [DOI] [Google Scholar]
- 12.Wardlaw JL, Gazzola KM, Wagoner A, Brinkman E, Burt J, Butler R. et al. Laser Therapy for Incision Healing in 9 Dogs. Front Vet Sci. 2018;5:349. doi: 10.3389/fvets.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Espinel-Ruperez J, Martin-Rios MD, Salazar V, Baquero-Artigao MR, Ortiz-Diez G. Incidence of surgical site infection in dogs undergoing soft tissue surgery: risk factors and economic impact. Vet Rec Open. 2019;6(1):e000233. doi: 10.1136/vetreco-2017-000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussbaum EL, Lilge L, Mazzulli T. Effects of 630-, 660-, 810-, and 905-nm laser irradiation delivering radiant exposure of 1-50 J/cm2 on three species of bacteria in vitro. J Clin Laser Med Surg. 2002;20(6):325–33. doi: 10.1089/104454702320901116. [DOI] [PubMed] [Google Scholar]
- 16.Baffoni M, Bessa LJ, Grande R, Di Giulio M, Mongelli M, Ciarelli A. et al. Laser irradiation effect on Staphylococcus aureus and Pseudomonas aeruginosa biofilms isolated from venous leg ulcer. Int Wound J. 2012;9(5):517–24. doi: 10.1111/j.1742-481X.2011.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gal P, Mokry M, Vidinsky B, Kilik R, Depta F, Harakalova M. et al. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med Sci. 2009;24(4):539–47. doi: 10.1007/s10103-008-0604-9. [DOI] [PubMed] [Google Scholar]
- 18.Pereira PR, de Paula JB, Cielinski J, Pilonetto M, Von Bahten LC. Effects of low intensity laser in in vitro bacterial culture and in vivo infected wounds. Rev Col Bras Cir. Jan-Feb 2014;41(1):49–55. doi: 10.1590/s0100-69912014000100010. [DOI] [PubMed] [Google Scholar]
- 19.Ranjbar R, Takhtfooladi MA. The effects of low level laser therapy on Staphylococcus aureus infected third-degree burns in diabetic rats. Acta Cir Bras. 2016;31(4):250–5. doi: 10.1590/S0102-865020160040000005. [DOI] [PubMed] [Google Scholar]
- 20.Percival SL, Francolini I, Donelli G. Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing. Future Microbiol. 2015;10(2):255–72. doi: 10.2217/fmb.14.109. [DOI] [PubMed] [Google Scholar]
- 21.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. Oct 1992;13(10):606–8. [PubMed] [Google Scholar]
- 22.Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther. 2006;19(6):383–90. doi: 10.1111/j.1529-8019.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 23.Lucroy MD, Edwards BF, Madewell BR. Low-intensity laser light-induced closure of a chronic wound in a dog. Vet Surg. 1999;28(4):292–5. doi: 10.1053/jvet.1999.0292. [DOI] [PubMed] [Google Scholar]
- 24.Kurach LM, Stanley BJ, Gazzola K, Fritz MC, Steficek BA, Hauptman JG. et al. The effect of Low-Level Laser Therapy on the Healing of Open Wounds in Dogs. Veterinary Surgery. 2015;44(8):988–996. doi: 10.1111/vsu.12407. [DOI] [PubMed] [Google Scholar]
- 25.Perego R, Proverbio D, Zuccaro A, Spada E. Low-level laser therapy: Case-control study in dogs with sterile pyogranulomatous pododermatitis. Vet World. 2016;9(8):882–7. doi: 10.14202/vetworld.2016.882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gammel JE, Biskup JJ, Drum MG, Newkirk K, Lux CN. Effects of low-level laser therapy on the healing of surgically closed incisions and surgically created open wounds in dogs. Vet Surg. May 2018;47(4):499–506. doi: 10.1111/vsu.12795. [DOI] [PubMed] [Google Scholar]
- 27.Bayat M, Vasheghani MM, Razavi N. Effect of low-level helium-neon laser therapy on the healing of third-degree burns in rats. J Photochem Photobiol B. 2006;83(2):87–93. doi: 10.1016/j.jphotobiol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Kaya GS, Kaya M, Gursan N, Kirecci E, Gungormus M, Balta H. The use of 808-nm light therapy to treat experimental chronic osteomyelitis induced in rats by methicillin-resistant Staphylococcus aureus. Photomed Laser Surg. 2011;29(6):405–12. doi: 10.1089/pho.2010.2807. [DOI] [PubMed] [Google Scholar]
- 29.Santos NR, de MSJB, Almeida PF, Ribeiro AA, Cangussu MC, dos Santos JN. et al. Influence of the combination of infrared and red laser light on the healing of cutaneous wounds infected by Staphylococcus aureus. Photomed Laser Surg. 2011;29(3):177–82. doi: 10.1089/pho.2009.2749. [DOI] [PubMed] [Google Scholar]
- 30.Silva DC, Plapler H, Costa MM, Silva SR, Sa Mda C, Silva BS. Low level laser therapy (AlGaInP) applied at 5J/cm2 reduces the proliferation of Staphylococcus aureus MRSA in infected wounds and intact skin of rats. An Bras Dermatol. 2013;88(1):50–5. doi: 10.1590/s0365-05962013000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZX, Kim SH. Effect of Photobiomodulation Therapy (660 nm) on Wound Healing of Rat Skin Infected by Staphylococcus. Photobiomodul Photomed Laser Surg. 2020;38(7):419–424. doi: 10.1089/photob.2019.4754. [DOI] [PubMed] [Google Scholar]
- 32.Krespi YP, Kizhner V. Laser-assisted nasal decolonization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus. Am J Otolaryngol. 2012;33(5):572–5. doi: 10.1016/j.amjoto.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Marchegiani A, Fruganti A, Spaterna A, Cerquetella M, Tambella AM, Paterson S. The Effectiveness of Fluorescent Light Energy as Adjunct Therapy in Canine Deep Pyoderma: A Randomized Clinical Trial. Vet Med Int. 2021;2021:6643416. doi: 10.1155/2021/6643416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min PK, Goo BL. 830 nm light-emitting diode low level light therapy (LED-LLLT) enhances wound healing: a preliminary study. Laser Ther. 2013;22(1):43–9. doi: 10.5978/islsm.13-or-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahamian FM, Goldstein EJ. Microbiology of animal bite wound infections. Clin Microbiol Rev. 2011;24(2):231–46. doi: 10.1128/CMR.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouro S, Vilela CL, Niza MM. Clinical and bacteriological assessment of dog-to-dog bite wounds. Vet Microbiol. 2010;144(1-2):127–32. doi: 10.1016/j.vetmic.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 38.de Sousa NT, Santos MF, Gomes RC, Brandino HE, Martinez R, de Jesus Guirro RR. Blue Laser Inhibits Bacterial Growth of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Photomed Laser Surg. 2015;33(5):278–82. doi: 10.1089/pho.2014.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karu T, Tiphlova O, Samokhina M, Diamantopoulos C, Sarantsev VP, Shveikin V. Effects of near-infrared laser and superluminous diode irradiation on Escherichia coli division rate. IEEE Journal of Quantum Electronics. 1990;26(12):2162–2165. [Google Scholar]
- 40.Kisuk K, Hun LD, Kun KS. Effects of low incident energy levels of infrared laser irradiation on the proliferation of streptococcus mutans. Laser Ther. 1992;4(2):81–85. [Google Scholar]
- 41.Nussbaum EL, Lilge L, Mazzulli T. Effects of 810 nm laser irradiation on in vitro growth of bacteria: comparison of continuous wave and frequency modulated light. Lasers Surg Med. 2002;31(5):343–51. doi: 10.1002/lsm.10121. [DOI] [PubMed] [Google Scholar]
- 42.Kolarova H, Nevrelova P, Tomankova K, Kolar P, Bajgar R, Mosinger J. Production of reactive oxygen species after photodynamic therapy by porphyrin sensitizers. Gen Physiol Biophys. 2008;27(2):101–5. [PubMed] [Google Scholar]
- 43.DeSimone NA, Christiansen C, Dore D. Bactericidal effect of 095-mW helium-neon and 5-mW indium-gallium-aluminum-phosphate laser irradiation at exposure times of 30, 60, and 120 seconds on photosensitized Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Phys Ther. Sep 1999;79(9):839–46. [PubMed] [Google Scholar]
- 44.Nussbaum EL, Lilge L, Mazzulli T. Effects of low-level laser therapy (LLLT) of 810 nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure. J Clin Laser Med Surg. 2003;21(5):283–90. doi: 10.1089/104454703322564497. [DOI] [PubMed] [Google Scholar]
- 45.Nussbaum EL, Mazzulli T, Pritzker KP, Heras FL, Jing F, Lilge L. Effects of low intensity laser irradiation during healing of skin lesions in the rat. Lasers Surg Med. 2009;41(5):372–81. doi: 10.1002/lsm.20769. [DOI] [PubMed] [Google Scholar]
- 46.Schindl A, Schindl M, Schon H, Knobler R, Havelec L, Schindl L. Low-intensity laser irradiation improves skin circulation in patients with diabetic microangiopathy. Diabetes Care. 1998;21(4):580–4. doi: 10.2337/diacare.21.4.580. [DOI] [PubMed] [Google Scholar]
- 47.Kubota J. Effects of diode laser therapy on blood flow in axial pattern flaps in the rat model. Lasers Med Sci. 2002;17(3):146–53. doi: 10.1007/s101030200024. [DOI] [PubMed] [Google Scholar]
- 48.Medrado AR, Pugliese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med. 2003;32(3):239–44. doi: 10.1002/lsm.10126. [DOI] [PubMed] [Google Scholar]
- 49.Larkin KA, Martin JS, Zeanah EH, True JM, Braith RW, Borsa PA. Limb blood flow after class 4 laser therapy. J Athl Train. 2012;47(2):178–83. doi: 10.4085/1062-6050-47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colombo F, Neto Ade A, Sousa AP, Marchionni AM, Pinheiro AL, Reis SR. Effect of low-level laser therapy (lambda660 nm) on angiogenesis in wound healing: a immunohistochemical study in a rodent model. Braz Dent J. 2013;24(4):308–12. doi: 10.1590/0103-6440201301867. [DOI] [PubMed] [Google Scholar]
- 51.Heu F, Forster C, Namer B, Dragu A, Lang W. Effect of low-level laser therapy on blood flow and oxygen- hemoglobin saturation of the foot skin in healthy subjects: a pilot study. Laser Ther. 2013;22(1):21–30. doi: 10.5978/islsm.13-or-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burger E, Mendes AC, Bani GM, Brigagao MR, Santos GB, Malaquias LC. et al. Low-level laser therapy to the mouse femur enhances the fungicidal response of neutrophils against Paracoccidioides brasiliensis. PLoS Negl Trop Dis. 2015;9(2):e0003541. doi: 10.1371/journal.pntd.0003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerdeira CD, Lima Brigagao MR, Carli ML, de Souza Ferreira C, de Oliveira Isac Moraes G, Hadad H. et al. Low-level laser therapy stimulates the oxidative burst in human neutrophils and increases their fungicidal capacity. J Biophotonics. 2016;9(11-12):1180–1188. doi: 10.1002/jbio.201600035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balsa IM, Culp WT. Wound Care. Vet Clin North Am Small Anim Pract. Sep 2015;45(5):1049–65. doi: 10.1016/j.cvsm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Stegemann MR, Coati N, Passmore CA, Sherington J. Clinical efficacy and safety of cefovecin in the treatment of canine pyoderma and wound infections. J Small Anim Pract. Jul 2007;48(7):378–86. doi: 10.1111/j.1748-5827.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 56.Six R, Cherni J, Chesebrough R, Cleaver D, Lindeman CJ, Papp G. et al. Efficacy and safety of cefovecin in treating bacterial folliculitis, abscesses, or infected wounds in dogs. J Am Vet Med Assoc. 2008;233(3):433–9. doi: 10.2460/javma.233.3.433. [DOI] [PubMed] [Google Scholar]
- 57. Bradley DS. Wounds. In: Riegel RJ, Godbold JC, eds. Laser Therapy in Veterinary Medicine: Photobiomodulation. John Wiley and Sons; 2017:100–113.
- 58.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomed Laser Surg. 2010;28(3):291–325. doi: 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 59.Araujo BF, Silva LI, Meireles A, Rosa CT, Gioppo NM, Jorge AS. et al. Effects of Low-Level Laser Therapy, 660 nm, in Experimental Septic Arthritis. ISRN Rheumatol. 2013;2013:341832. doi: 10.1155/2013/341832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasheghani MM, Bayat M, Dadpay M, Habibie M, Rezaei F. Low-level laser therapy using 80-Hz pulsed infrared diode laser accelerates third-degree burn healing in rat. Photomed Laser Surg. 2009;27(6):959–64. doi: 10.1089/pho.2008.2366. [DOI] [PubMed] [Google Scholar]
- 61.Houreld N, Abrahamse H. Houreld N, Abrahamse HIrradiation with a 6328 nm helium-neon laser with 5 J/cm2 stimulates proliferation and expression of interleukin-6 in diabetic wounded fibroblast cells. Diabetes Technol Ther. 2007;9(5):451–9. doi: 10.1089/dia.2007.0203. [DOI] [PubMed] [Google Scholar]
- 62.Gagnon D, Gibson TW, Singh A, zur Linden AR, Kazienko JE, LaMarre J. An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model. BMC Vet Res. 2016;12:73. doi: 10.1186/s12917-016-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novaes RD, Goncalves RV, Cupertino MC, Araujo BM, Rezende RM, Santos EC. et al. The energy density of laser light differentially modulates the skin morphological reorganization in a murine model of healing by secondary intention. Int J Exp Pathol. 2014;95(2):138–46. doi: 10.1111/iep.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 2009;27(2):221–6. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- 65.Ankri R, Lubart R, Taitelbaum H. Estimation of the optimal wavelengths for laser-induced wound healing. Lasers Surg Med. 2010;42(8):760–4. doi: 10.1002/lsm.20955. [DOI] [PubMed] [Google Scholar]
- 66.Krespi YP, Kizhner V, Kara CO. Laser-induced microbial reduction in acute bacterial rhinosinusitis. Am J Rhinol Allergy. 2009;23(6):e29–32. doi: 10.2500/ajra.2009.23.3404. [DOI] [PubMed] [Google Scholar]
- 67.Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD. Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis. Photochem Photobiol. 2010;86(2):476–9. doi: 10.1111/j.1751-1097.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 68.Hashmi JT, Huang YY, Sharma SK, Kurup DB, De Taboada L, Carroll JD. et al. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42(6):450–66. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]