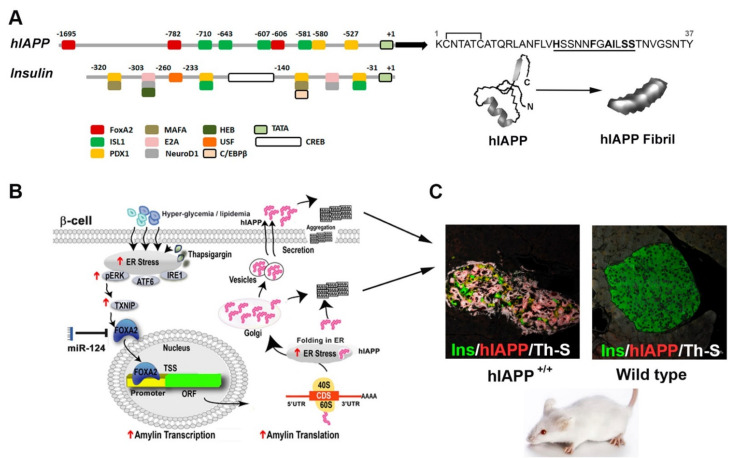
Figure 1.
Molecular determinants of hIAPP synthesis, aggregation and toxicity in pancreatic islets. (A) Diagram depicts main transcriptional regulatory sites and factors from hIAPP and insulin promoters. Primary sequence of fully processed mature hIAPP form is shown on the right. Amyloidogenic region in hIAPP amino acids sequence is underlined. AFM micrograph of a single fibril self-assembled from mature synthetic hIAPP monomers is shown below. Adapted from Reference [46]. (B) Diagram depicts main steps in hIAPP synthesis in glucose-challenged or ER-stressed pancreatic β-cells, including activation of a central TXNIP/FOXA2-mediated signaling pathway. Following processing, hIAPP is stored together with insulin in secretory vesicles. Disproportionate production and/or processing of hIAPP in human islets may initiate its aggregation and consequently β-cell stress and islet amyloidosis. (C) Excessive intracellular and/or extracellular accumulation of protein aggregates in hIAPP transgenic mice induces a loss in β-cell mass and hyperglycemia, which are main pathological attributes of T2DM. Note a decrease in insulin levels (green) with simultaneous accumulation of hIAPP (red) and thioflavin T (ThT)-positive protein aggregates (white) in hIAPP transgenic mouse islets as compared to wild-type mice islets which are hIAPP- and aggregate-free. Additionally, note severe islet cells atrophy and distortion of hIAPP transgenic mouse islets as compared to morphologically and functionally preserved islets from non-diabetic wild-type mice. Confocal micrographs adapted from Reference [33].