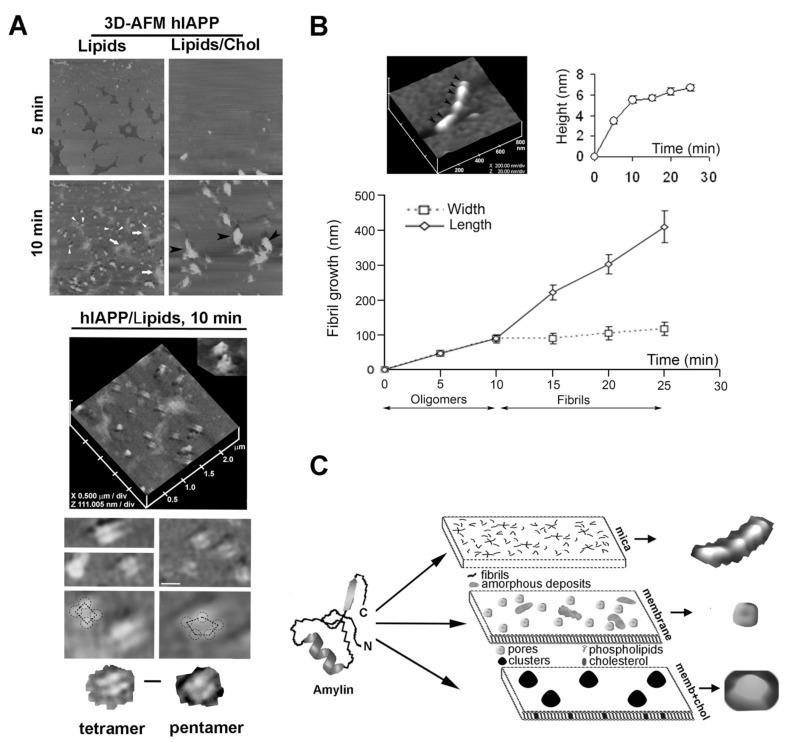
Figure 4.
Polymerization pathways and polymorphic structures of hIAPP on different surfaces. (A) Time-lapse 3D-AFM analysis of hIAPP aggregation on soft negatively charged lipid/sterol planar membranes. Freshly dissolved monomeric hIAPP was added to preformed planar lipidic membranes of different composition, and the modulatory effect of lipids and cholesterol on the extent, size, organization and morphology of amylin aggregates was analyzed by AFM. Upper micrographs, 5 × 5 μm. At higher magnification (2.5 × 2.5 μm), single highly ordered hIAPP tetrameric and pentameric oligomeric assemblies are resolved (lower insets; scale bar, 100 nm), two of which feature a central pore (hIAPP/Lipids, 10 min, top inset). Tetrameric (lower left micrograph) and pentameric (lower right micrograph) subunits of individual hIAPP supramolecular complexes are outlined for clarity (scale bar, 50 nm). (B) Time-lapse 3D-AFM analysis of hIAPP aggregation on stiff mica surface. In contrast to planar membranes, self-assembled hIAPP oligomers (black arrowheads, AFM micrograph) bi-directionally extended into a mature fibril. hIAPP polymerization on mica was visualized and quantified with time-lapse AFM and 3D-section analysis, which revealed the width, length and height of full-grown hIAPP fibrils and their intermediates. (C) Structural diversity of hIAPP polymorphic forms on different surfaces. AFM micrographs of a single fibril, a pore and cluster self-assembled from hIAPP monomers on different surfaces are presented for clarity. AFM micrographs and fibril growth curve were adapted from References [46,84].