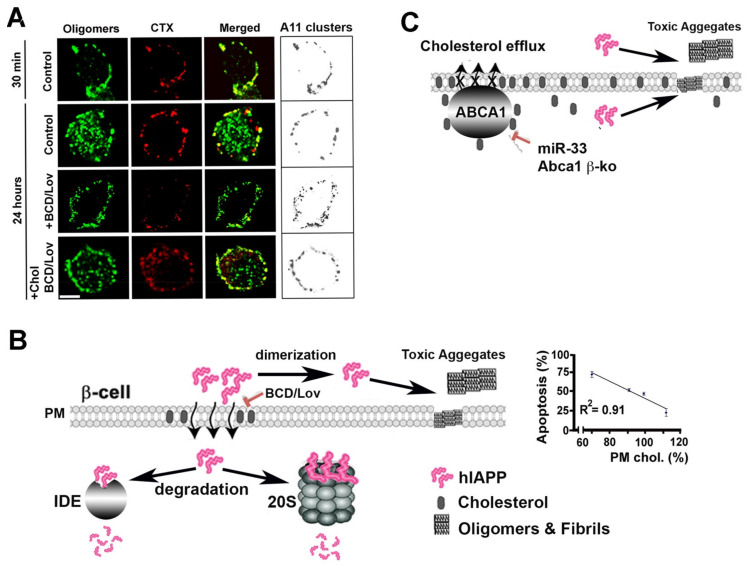
Figure 5.
Cholesterol controls hIAPP trafficking, aggregation and toxicity in pancreatic islets. (A) Time-lapse laser scanning confocal microscopy (LSCM) analysis of hIAPP oligomerization and internalization in cultured human islets. Freshly dissolved hIAPP was incubated with cholera toxin (CTX) in acute human islets for indicated periods of time, in the presence or absence of cholesterol depleting agents, and the subcellular distribution, size and accumulation of A11-positive hIAPP oligomeric clusters was quantified by confocal microscopy and a conformation specific anti-oligomer A11 antibody. Bar, 5 μm. Organization and plasma membrane distribution of hIAPP oligomers (clusters) prior to and following depletion of PM cholesterol are shown on the right (boxes). LSCM micrographs adapted from Reference [66]. (B) Intact cholesterol organization on PM is required for internalization of hIAPP soluble oligomeric assemblies. Following internalization, hIAPP monomeric and oligomeric structures are targeted for degradation by 20S proteasome complex and intracellular proteolytic enzymes, such as insulin-degrading enzyme (IDE) [71,79]. Cholesterol depleting agents, betacyclodextrin (BCD) and lovostatin (Lov), disturb cholesterol homeostasis, leading to less hIAPP clearance and, consequently, its enhanced oligomerization and aggregation in solution and on the cell surface. Graph depicts inverse relationship between PM cholesterol content and hIAPP toxicity in human islets. Graph adapted from Reference [66]. (C) Disruption of cholesterol efflux in hIAPP-transgenic rodent islets stimulate hIAPP aggregation, islet amyloidosis and β-cell dysfunction. Β-cell-specific downregulation of cholesterol-specific ATP-binding cassette transporter 1 (ABCA1) was achieved by using knockout and RNAi-silencing approaches [109].