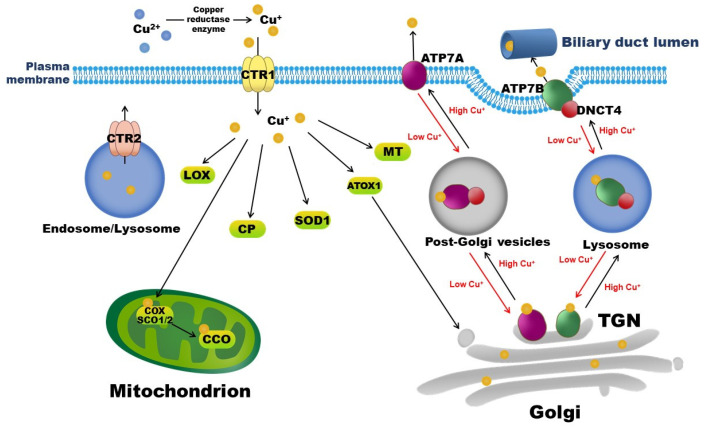
Figure 1.
Copper-binding proteins and intracellular copper transportation. Copper is exclusively absorbed by enterocytes in the small intestine via CTR1. CTR2 is a low-affinity copper importer that localizes to endosomes and lysosomes. Intracellular copper-binding proteins include COX, SCO, SOD, MT, CP, and LOX. ATP7A and ATP7B are copper exporters. Under normal conditions, ATP7A and ATP7B localize to TGN, where they supply copper to copper-dependent enzymes in the secretory pathway. When the cytosolic copper level rises, ATP7A or ATP7B interacts with DNCT4 and traffics to endosome-like vesicles and then to the plasma membrane, pumping excess copper into the extracellular space, or into bile in the case of the liver, to reduce intracellular copper levels. By contrast, when the intracellular copper level is low, ATP7A or ATP7B recycles to the TGN and transports copper from the cytoplasm into the Golgi. ATOX1: antioxidant 1 copper chaperone; ATP7A: copper-transporting ATPase 1; ATP7B: copper-transporting ATPase 2; CTR1: copper transporter 1; CTR2: copper transporter 2; CCO: cytochrome c oxidase; COX: cytochrome c oxidase copper chaperone; CP: ceruloplasmin, DNCT4: p62 subunit of dynactin; LOX: lysyl oxidase; MT: metallothionein; SCO: synthesis of cytochrome c oxidase; SOD: superoxide dismutase; TGN: trans-Golgi network.