Abstract
Since the synthesis of prontosil the first prodrug shares their chemical moiety, sulfonamides exhibit diverse modes of actions to serve as antimicrobials, diuretics, antidiabetics, and other clinical applications. This inspiring chemical nucleus has promoted several research groups to investigate the synthesis of new members exploring new clinical applications. In this study, a novel series of 5(4H)-oxazolone-based-sulfonamides (OBS) 9a–k were synthesized, and their antibacterial and antifungal activities were evaluated against a wide range of Gram-positive and -negative bacteria and fungi. Most of the tested compounds exhibited promising antibacterial activity against both Gram-positive and -negative bacteria particularly OBS 9b and 9f. Meanwhile, compound 9h showed the most potent antifungal activity. Moreover, the OBS 9a, 9b, and 9f that inhibited the bacterial growth at the lowest concentrations were subjected to further evaluation for their anti-virulence activities against Pseudomonas aeruginosa and Staphylococcus aureus. Interestingly, the three tested compounds reduced the biofilm formation and diminished the production of virulence factors in both P. aeruginosa and S. aureus. Bacteria use a signaling system, quorum sensing (QS), to regulate their virulence. In this context, in silico study has been conducted to assess the ability of OBS to compete with the QS receptors. The tested OBS showed marked ability to bind and hinder QS receptors, indicating that anti-virulence activities of OBS could be due to blocking QS, the system that controls the bacterial virulence. Furthermore, anticancer activity has been further performed for such derivatives. The OBS compounds showed variable anti-tumor activities, specifically 9a, 9b, 9f and 9k, against different cancer lines. Conclusively, the OBS compounds can serve as antimicrobials, anti-virulence and anti-tumor agents.
Keywords: oxazolone, sulfonamide, antimicrobial, anti-virulence, antibiofilm, anticancer
1. Introduction
The increasing pervasiveness of microbial resistance represents a major issue globally. Despite the discovery and significant development of several new antibiotics, multidrug-resistant bacteria are still becoming more prevalent, and are creating a serious public health risk for the population [1,2]. It has been estimated that 700,000 people in the world die every year from antibiotic-resistant infectious bacterial diseases. In the absence of new prevention or treatment remedies, by 2050, it is estimated that 10 million people worldwide will die of these infectious diseases each year [3]. Consequently, the development of a new, powerful therapeutic approach to treat and kill Gram-negative, as well as Gram-positive, human pathogens is urgently needed. It is well-recognized that antibiotics-resistant bacterial infections are not due to free bacteria but rather to bacteria existing within a biofilm [4]. The resistance of biofilm-forming bacteria to conventional antimicrobials is attributed to: (1) the failure of the antimicrobial to penetrate the biofilm, (2) the evolution of complex drug resistance properties, and (3) biofilm mediated inactivation or modification of antimicrobial enzymes [5].
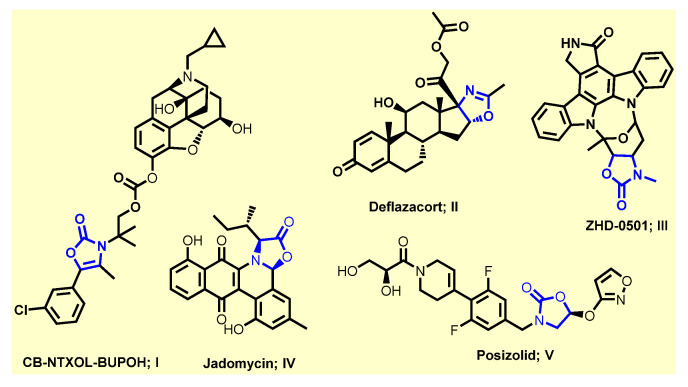
Conversely, heterocycles are a significant class of cyclic compounds that are considered the prominent source of biologically active compounds due to their diverse structures [6,7,8]. Among them, oxazol-5-(4H)-one (2,4-disubstituted 5-oxo-4,5-dihydro-1,3-oxazole, also known as azalactone or oxazolone) is one of the large varieties of interesting molecules with numerous applications in chemistry and biology [9,10]. The exocyclic double bond in position four of the oxazolone ring provides a new reactivity that allows the construction of interesting derivatives [10]. Moreover, 2-phenyloxazol-5(4H)-ones with an additional exocyclic double bond exhibits a wide range of biological activities such as antibacterial [11], immunomodulatory [10,12,13], antidiabetic [14], antiviral [15], antifungal [16], anticancer [17], anti-inflammatory [18], anti-HIV [19], anti-angiogenic [20], sedative [21], and tyrosinase inhibitory activities [22], among others (Figure 1). Notably, there are numerous drugs containing oxazolone motif in their structure such as the carbonate codrug, CB-NTXOL-BUPOH (I), consisting of 6-β-naltrexol (the major active metabolite of naltrexone, a potent μ-opioid receptor antagonist used in the treatment of alcohol dependence and opioid abuse) covalently linked by carbonate ester linkage to a modified form of hydroxybupropion (bupropion with oxazolone) [23]. Deflazacort (II) has anti-inflammatory and immunosuppressive effects [24]. ZHD-0501 (III) is a metabolite of staurosporine (STA) analog with an oxazolone scaffold, which inhibits the proliferation of several human and murine cancer cell lines [25]. Jadomycin (IV) is an antifungal with a unique 8H-benz[b]oxazolo [3,2-] phenanthridine pentacyclic skeleton produced by the bacterium Streptomyces venezuelae ISP 5230 [26]. Posizolid (V) is an oxazolidinone antibiotic under investigation by AstraZeneca for the treatment of bacterial infections [27]. Moreover, oxazolone scaffold is an attractive heterocyclic precursor which can be used as versatile building blocks in organic synthesis, as they consist of “masked” amino acids and contain numerous reactive sites allowing a diversity of possible modifications. Their reactivity (nucleophilic attack to the carbon atom at position five of the oxazolone ring) makes them excellent substrates for the preparation of structurally complex amino acids and highly substituted heterocycles, enol acetate and benzoxazinone derivatives, phenylpyruvic acid, imidazolinones, amino acids, amino alcohols, amides, dyes and triazinones [9,10,28]. The azlactone transformations have allowed facile access to natural compounds and pharmaceutically and biologically intriguing molecules.
Figure 1.
Some drugs containing oxazolone scaffold.
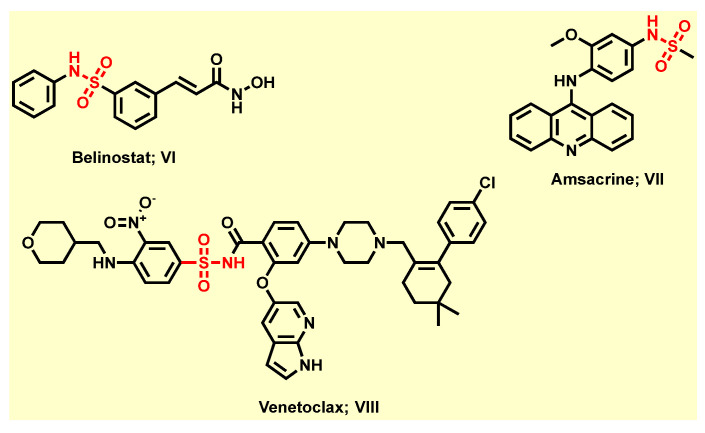
Furthermore, sulfonamide derivatives have evoked high favor and constitute privileged structural motifs in medicinal chemistry because they exhibit a wide range of pharmacological activities [8] including anticancer [29,30], antibacterial [31], anti-tuberculosis [32], anti-carbonic anhydrase [33], anti-fungal [34], anti-inflammatory [35], anti-diabetic [36], antiviral [37], anti-oxidant [38], diuretic [36], antimalarial [39], and antithyroid [36], in addition to protease inhibitory activity in vitro and in vivo, among others [36]. Obviously, some sulfonamide derivatives have been approved by FDA for cancer therapy. For instance, the third approved histone deacetylase (HDAC) inhibitor, Belinostat (VI), is approved to treat T-cell lymphoma after Vorinostat and Romidepsin (Figure 2) [40]. The topoisomerase II inhibitor, Amsacrine (VII), is approved to treat acute leukemias and malignant lymphomas through intercalating into the DNA of tumor cells (Figure 2) [41]. Additionally, the highly selective Bcl-2 inhibitor, Venetoclax (VIII), is now approved for treatment of chronic lymphocytic leukemia (CLL) patients with a 17p chromosomal deletion who have received at least one prior therapy (Figure 2) [42,43]. Moreover, sulfonamide moiety is usually considered as an effective bioisostere of the carboxylic group because the distance between two oxygen atoms is about similar in these two functional groups [44,45]. Therefore, sulfonamide motif could be engaged in a network of hydrogen bonds which are the same as the carboxylic group with fewer drawbacks of the carboxylic group, such as metabolic instability, toxicity, and limited passive diffusion across biological membranes [44,45].
Figure 2.
Selected FDA-approved anti-cancer agents containing sulfonamide moiety.
Motivated by the above information and based on bacterium being linked to cancer by two mechanistic pathways—induction of chronic inflammation and production of carcinogenic bacterial metabolites [46]—a series of new 5(4H)-oxazolone-benzene sulfonamide derivatives were designed, synthesized and evaluated for their antibacterial, antifungal, antibiofilm, anti-virulence and anticancer activities. Moreover, a molecular docking study was carried out to investigate the binding mode and interaction of the most potent derivatives into the active site of Pseudomonas aeruginosa quorum sensing (QS) receptor (PDB: 1ROS) that orchestrates the bacterial virulence [47,48].
2. Results and Discussion
2.1. Chemistry
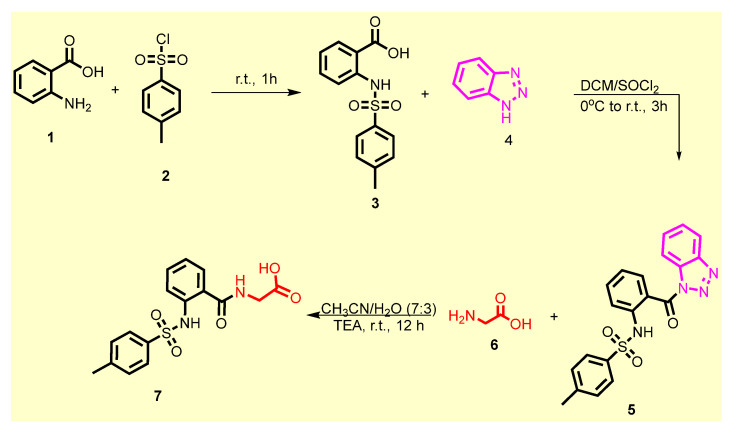
The chemical synthetic approach of the target compounds 9a–k is outlined in Scheme 1 and Scheme 2 As illustrated in Scheme 1. 4-Toluenesulfonyl anthranilic 3 was synthesized according to the reported protocol via nucleophilic substitution reaction of anthranilic acid 1 with p-toluensulfonyl chloride 2 in the presence of sodium hydroxide [47]. Compound 3 was subjected to the same reaction with 1H-benzotriazole 4 in DCM using thionyl chloride at room temperature to afford the benzene sulfonamide 5 in excellent yield. The latter compound was further treated with glycine 6 in acetonitrile/H2O under basic conditions to furnish (2-((4-methylphenyl)sulfonamido)benzoyl) glycine 7 (Scheme 1).
Scheme 1.
Synthesis of acid 7.
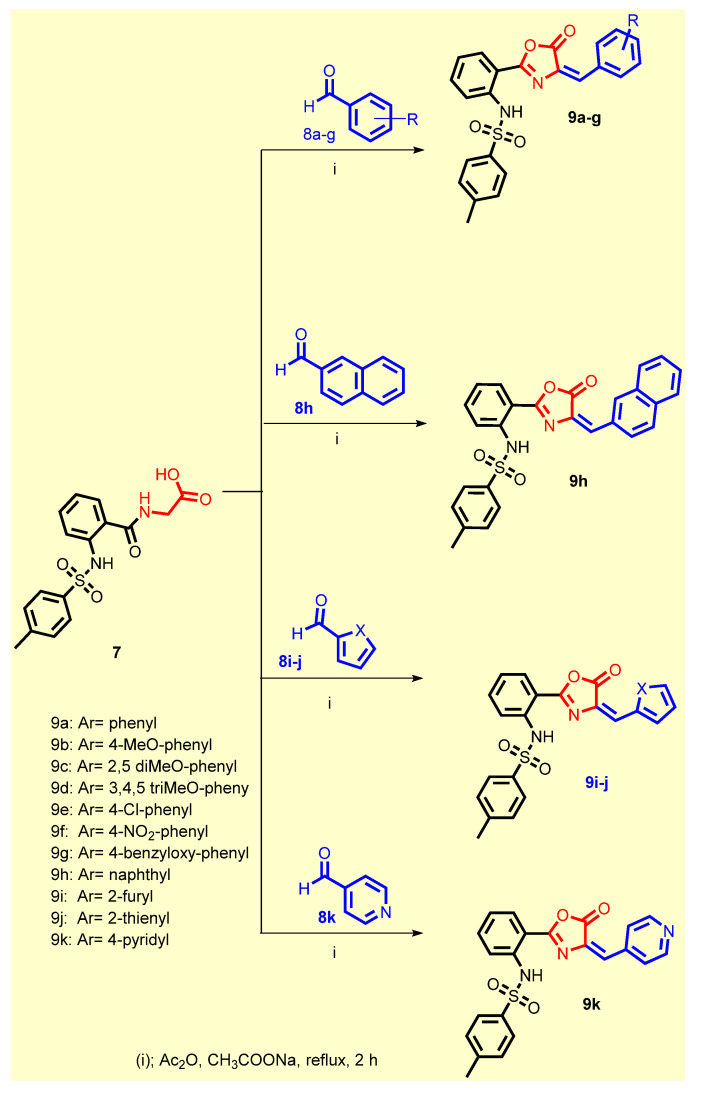
Scheme 2.
Synthesis of the target compounds 9a–k.
The former acid 7 was reacted with the appropriate aldehydes 8a-j using acetic anhydride in the presence of anhydrous sodium acetate to give the desired oxazolone-benzenesulfonamide derivatives 9a–k (Scheme 2). All the synthesized compounds were in accordance with their expected structures which have been elucidated by various spectroscopic techniques such as 1H NMR and 13C NMR spectra and elemental analyses (see Supplementary Materials).
2.2. Biological Screening
2.2.1. In Vitro Antimicrobial Activity
Minimum Inhibitory Concentrations (MICs) of Synthesized Compounds against Different Gram-Positive and -Negative Bacteria
In order to evaluate the antimicrobial activities of synthesized compounds, their MICs were determined against different Gram-positive and -negative bacteria, and fungi (Table 1). Most of the tested compounds exhibited promising antibacterial activity. Compounds 9a, 9b, 9c, 9e and 9f were the most active derivatives with broad spectrum of activity against Gram-positive and Gram-negative bacteria. Among them, 9a (with unsubstituted phenyl group), 9b (4-methoxy) and 9f (4-NO2) were the most potent. The result of antifungal activity screening showed that most of the tested derivatives had moderate, or weak activity, or were inactive against the used fungal strains, as illustrated in Table 1. Ongoing throughout the details, 9a (with unsubstituted phenyl group) showed a broad spectrum of activity against all bacterial strains, in particular against Escherichia coli. However, 9a had weak antifungal activity against Aspergillus niger and moderate antifungal activity against Candida albicans.
Table 1.
Minimum inhibitory concentration (MIC) by µg/mL against standard strains of different microorganisms.
| Compound | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Staphylococcus aureus ATCC 6538 |
Staphylococcus epidermidis ATCC 12228 |
Micrococcus spp. ATCC 10240 |
Pseudomonas aeruginosae ATCC 47085 |
Klebsiella pneumoniae ATCC 27736 |
Salmonella typhimurium ATCC 14028 |
Escherichia coli ATCC 10536 |
Aspergillus niger ATCC 16404 |
Candida albicans ATCC 10231 | |
| 9a | 4 | 4 | 4 | 8 | 4 | 4 | 2 | 32 | 16 |
| 9b | 2 | 1 | 1 | 4 | 4 | 4 | 2 | >32 | >32 |
| 9c | 8 | 4 | 4 | 16 | 8 | 8 | 4 | 8 | 4 |
| 9d | 4 | 2 | 2 | 32 | 16 | 16 | 4 | >32 | >32 |
| 9e | 2 | 2 | 2 | 8 | 2 | 4 | 4 | 16 | 8 |
| 9f | 2 | 2 | 2 | 4 | 2 | 2 | 4 | >32 | >32 |
| 9g | 2 | 2 | 2 | 16 | 8 | 16 | 2 | >32 | >32 |
| 9h | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 4 | 2 |
| 9i | 2 | 2 | 2 | >32 | >32 | >32 | >32 | >32 | >32 |
| 9j | 16 | 8 | 8 | >32 | >32 | >32 | >32 | >32 | >32 |
| 9k | 4 | 4 | 2 | >32 | >32 | >32 | 16 | >32 | >32 |
| Amoxycillin | 0.5 | 0.5 | 0.25 | 2 | 1 | 1 | 0.5 | - | - |
| Cefotaxime | 0.5 | 0.25 | 0.25 | 1 | 0.5 | 1 | 0.125 | - | - |
| Sulphamethoxazole | 2 | 2 | 1 | 4 | 2 | 2 | 1 | - | - |
| Nystatin | - | - | - | - | - | - | - | 4 | 4 |
Replacement of hydrogen with the electron donating methoxy group, i.e., OBS 9b, resulted in a 2-fold increase in the activity against Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus spp. and Pseudomonas aeruginosae and retained the same activity as OBS 9a against Klebsiella pneumonia, Salmonella typhimurium and Escherichia coli. Introduction of 2,5-dimethoxy group, as in OBS 9c, retained the same activity against S. epidermidis, and Micrococcus spp. with a 2-fold decrease against S. aureus, P. aeruginosa, K. pneumonia, S. typhimurium and E. coli. Replacement of hydrogen with trimethoxy groups, i.e., OBS 9d, retained the same activity as 9a against S. aureus and improved (2-fold) activity against S. epidermidis and Micrococcu89s spp. against compound 9a. Moreover, compound 9d exhibited a slight decrease in activity (2-fold) against E. coli and a marked decrease in the antibacterial activity against P. aeruginosa, K. pneumonia, and S. typhimurium. Introduction of the weakly deactivating Cl group, as in compound 9e, retained the broad spectrum of activity against all Gram-positive and -negative bacteria with improved potency (2-fold increase in activity) against all Gram-positive organisms: S. epidermidis, and Micrococcus spp. Additionally, compound 9e displayed more potency (2-fold increase) than 9a against K. pneumonia and showed the same activity as 9a against P. aeruginosa and S. typhimurium, while showing a 2-fold decrease in activity than 9a against E. coli. Introduction of the strongly electron withdrawing NO2 group as in compound 9f, retained the broad spectrum of activity against Gram-positive and -negative organisms with improved potency (2-fold) against all strains except against E. coli (2-fold decrease in activity than 9a). Replacement of phenyl group with benzyloxy group (9g) resulted in an improvement of antibacterial activity against all Gram-positive organisms, S. epidermidis, and Micrococcus spp. Although OBS 9g showed the same antibacterial activity as compound 9a against the Gram-negative organism E. coli, it exhibited moderate antibacterial activity against K. pneumonia and weak antibacterial activity against P. aeruginosae and S. typhimurium. Replacement of phenyl group with naphthyl group (9h) resulted in a loss of activity against all bacterial strains but surprisingly displayed the highest antifungal activity against Aspergillus niger and Candida albicans with MIC 8 and 4 µg/mL, respectively. Replacement of naphthyl group in 9h with heterocyclic moieties led to retaining the activity against Gram-positive organisms only. On comparing these derivatives with compound 9a, we noticed that introducing 2-furyl moiety (OBS 9i) led to a 2-fold increase against the three test Gram-positive strains S. aureus, S. epidermidis, and Micrococcus spp. Installment of 2-thienyl group (9j) instead of the phenyl group displayed a decrease in the activity of nearly 4-fold against Staphylococcus aureus and 2-fold against S. epidermidis, Micrococcus spp. Shifting from phenyl group to 2-pyridyl group (9k) led to an increase in the antibacterial activity by 2-fold against Micrococcus spp. and retained the same activity as in compound 9a against S. aureus and S. epidermidis.
Antifungal Activity of Synthesized Compounds
Notably, compound 9h exhibited potent antifungal activity with MIC 4 and 2 µg/mL against Aspergillus niger and Candida albicans, respectively. Compound 9c was second in potency compared with 9h, and showed moderate antifungal activity with MIC 8 and 4 µg/mL against Aspergillus niger and Candida albicans, respectively. Compound 9k showed moderate activity against Candida albicans and was weak against Aspergillus niger with MIC 8 and 16 µg/mL, respectively. Compound 9a displayed weak antifungal activity against Candida albicans and very weak antifungal activity against Aspergillus niger with MIC 16 and 32 µg/mL, respectively. The rest of the compounds were inactive as antifungal agents, with MIC more than 32 µg/mL.
Antibiofilm Activity of Synthesized Compounds
Prior to the investigation of the anti-biofilm and anti-virulence activities of tested compounds, the effect of compounds at sub-MIC (=½ MIC) on P. aeruginosa and S. aureus growth was evaluated to exclude any effect on bacterial growth [49,50]. There were no significant differences between bacterial growth in the presence or absence of tested compounds at sub-MIC. It is worth mentioning that the sub-MIC concentrations of tested compounds are used in all the next experiments.
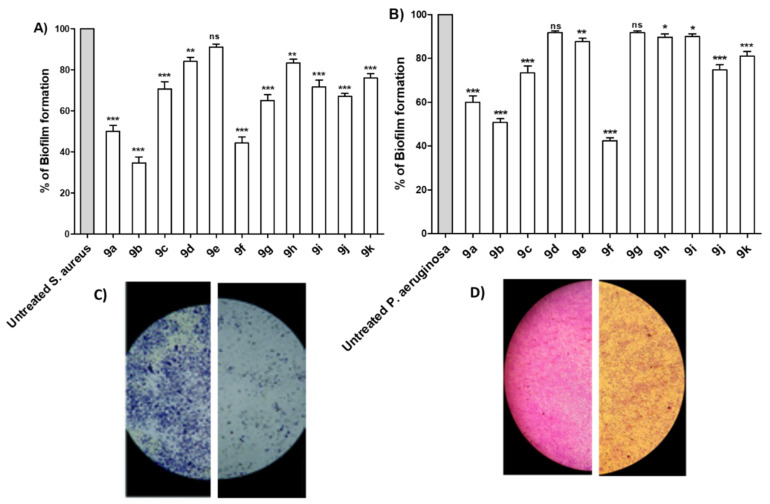
Bacterial virulence is regulated via a quorum sensing (QS) system in an inducer/receptor manner [51,52]. Diminishing bacterial virulence is an advantageous strategy to decrease the development of bacterial resistance [47,53,54,55,56]. In this direction, anti-virulence and anti-QS activities have been explored in several studies as reviewed [51]. The ability of P. aeruginosa or S. aureus to form biofilms was assayed in the absence or presence of tested compounds at sub-MIC. Significantly, most of the compounds were able to reduce the formation of biofilm, especially compounds 9a, 9b and 9f (Figure 3A,B). The experiment was conducted in triplicate and a one-way ANOVA test was employed to test the statistical significance using Graphpad Prism 8 software. The results were significant statistically where p values < 0.05. Moreover, microscopic visualization of biofilm under the effect of tested compounds was also performed by light microscopy. Representative images for the inhibitory effects on S. aureus and P. aeruginosa biofilm formation are shown (Figure 3C,D). The microscopic images show a marked reduction in both the thickness of and surface area covered by the biofilms in presence of the tested compound.
Figure 3.
Antibiofilm activities of the synthesized compounds. The antibiofilm activities of the synthesized compounds were evaluated at their sub-MIC concentrations to avoid any influence on the bacterial growth. The crystal violet method was used to quantify the inhibition activity of compounds on (A) S. aureus and (B) P. aeruginosa. The synthesized compounds showed variable abilities to inhibit the biofilm formation, however, compounds 9a, 9b and 9f showed the highest abilities to inhibit biofilm formation in both tested bacterial strains. Representative image for the inhibitory effect of compound 9e on the biofilm formation by (C) S. aureus and (D) P. aeruginosa were taken. The formed biofilms were markedly reduced showing scattered thinner layers of bacterial biofilms. (*** = p < 0.001; ** = p < 0.01; * p < 0.05; ns = non-significant).
Tested Compounds Diminish the Production of Bacterial Virulence Extracellular Enzymes
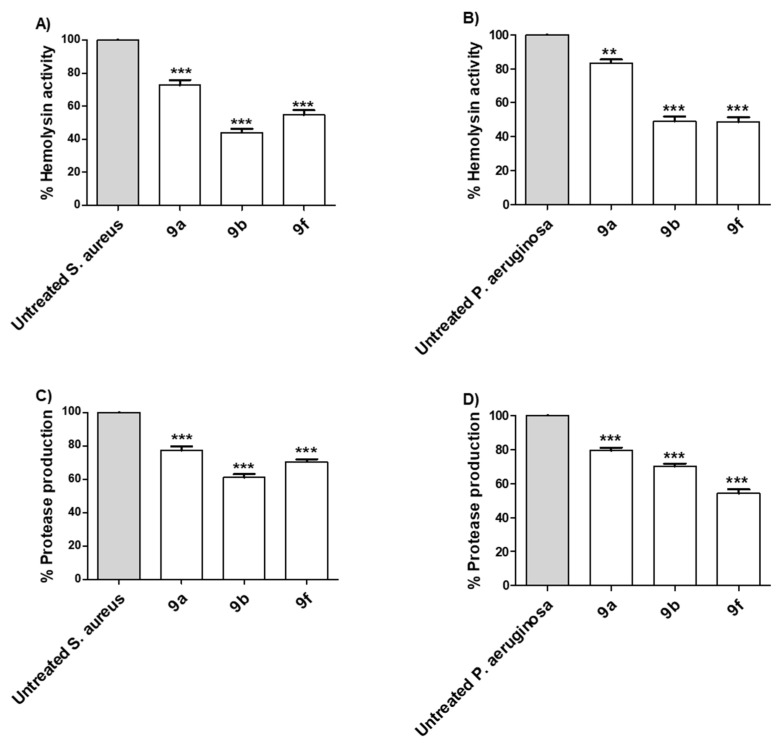
Bacteria establish their infection into the host cells by utilizing a diverse arsenal of virulence factors [57,58]. Extracellular enzymes play crucial roles in the bacterial invasion and spread, for instance, protease and hemolysins [48,53,54]. Herein, the proteolytic and hemolytic activities of selected compounds 9a, 9b and 9f were assayed in P. aeruginosa and S. aureus (Figure 4). The tested compounds 9a, 9b and 9f at sub-MIC showed significant ability to diminish the production of extracellular enzymes. The experiments were conducted in triplicate and the significance was analyzed using one-way ANOVA (Graphpad Prism 8 software). The results were significant statistically where p values <0.05. The results were presented as the percentage of inhibition from untreated bacterial.
Figure 4.
Selected active synthesized compounds decreased the production of extracellular enzymes. The anti-virulence activities were assessed for the selected compounds 9a, 9b and 9f at their sub-MIC against P. aeruginosa and S. aureus. The three tested compounds significantly diminished the hemolytic activity of (A) S. aureus and (B) P. aeruginosa. Moreover, the tested compounds significantly reduced the production of protease of (C) S. aureus and (D) P. aeruginosa. (*** = p < 0.001, ** = p < 0.01).
Tested Compounds Diminish the Production of Bacterial Virulence Factors
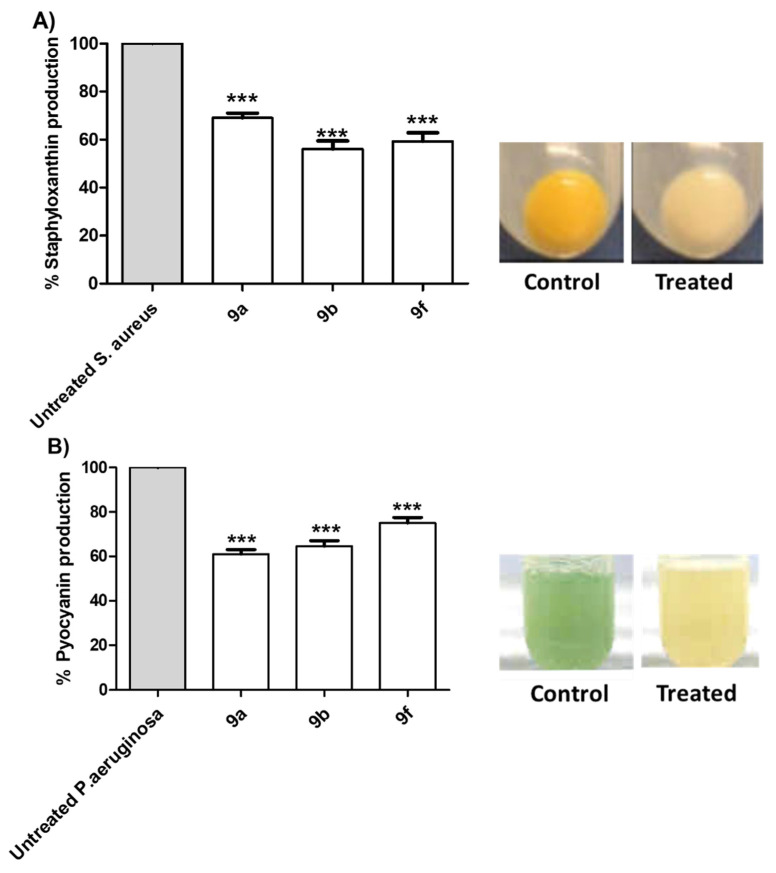
S. aureus pigment staphyloxanthin is an important virulence factor due to antioxidant action that helps in overcoming the host immune defense [59]. Additionally, pyocyanin has emerged as an important virulence factor produced by P. aeruginosa [47,48]. The inhibitory effects of tested compounds 9a, 9b and 9f at sub-MIC on the production of bacterial virulence factors staphyloxanthin in S. aureus and pyocyanin in P. aeruginosa were evaluated (Figure 5). The tested compounds showed a significant ability to reduce the production of bacterial pigments. The experiments were conducted in triplicate and the significance was analyzed using one-way ANOVA (Graphpad Prism 8 software). The results were significant statistically where p values < 0.05.
Figure 5.
Anti-virulence activities of the active synthesized compounds. Selected compounds 9a, 9b and 9f at sub-MIC were tested for their ability to decrease the production of QS-controlled bacterial virulence factors. The tested compounds significantly decreased the production of (A) S. aureus pigment staphyloxanthin and (B) P. aeruginosa pigment pyocyanin. (*** = p < 0.001).
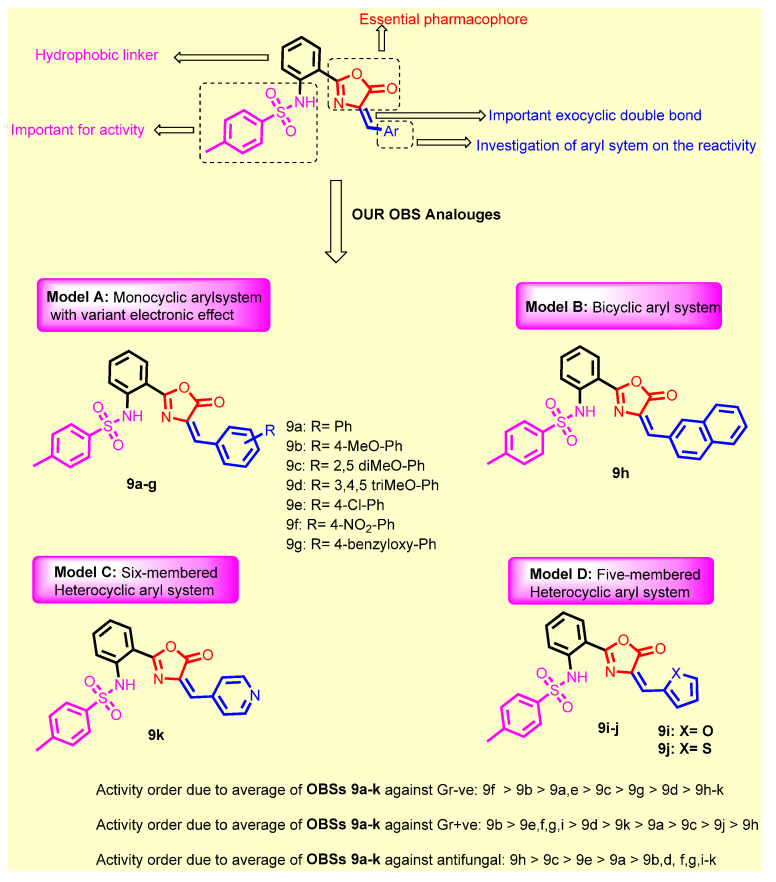
SAR Study
SAR of the synthesized candidates are summarized as follows (Figure 6):
Introduction of 4-methoxy group (9b) resulted in a 2-fold increase in the activity against Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus spp. and Pseudomonas aeruginosae, while retaining the same activity as compound 9a against Klebsiella pneumonia, Salmonella typhimurium and Escherichia coli;
Introduction of 2,5-dimethoxy group (9c) retained the same activity against Staphylococcus epidermidis and Micrococcus spp., and a 2-fold decrease against Staphylococcus aureus, Pseudomonas aeruginosae, Klebsiella pneumonia, Salmonella typhimurium and Escherichia coli;
Replacement of hydrogen with trimethoxy groups (9d) resulted in the same activity against Staphylococcus aureus, improved activity (2-fold) against Staphylococcus epidermidis and Micrococcus spp., slightly decreased activity (2-fold) against Escherichia coli, and significantly decreased the antibacterial activity against Pseudomonas aeruginosae, Klebsiella pneumonia, and Salmonella typhimurium;
Introduction of the weakly deactivating Cl group (9e) improved potency (2-fold increase in activity) against all Gram-positive organisms, Staphylococcus epidermidis, and Micrococcus spp., improved potency (2-fold increase) against Klebsiella pneumonia, resulted in the same activity against Pseudomonas aeruginosae and Salmonella typhimurium, and a 2-fold decrease in activity against Escherichia coli;
Introduction of the strongly activating NO2 group (9f) improved potency (2-fold) against all strains except against Escherichia coli (2-fold decrease);
Replacement of phenyl group with benzyloxy group (9g) led to the improvement of antibacterial activity against all Gram-positive organisms, the same antibacterial activity against the Gram-negative organism Escherichia coli, moderate antibacterial activity against Klebsiella pneumonia, and weak antibacterial activity against Pseudomonas aeruginosae and Salmonella typhimurium;
Replacement of phenyl group with naphthyl one (9h) resulted in loss of activity against all bacterial strains, whereas it displayed the highest antifungal activity against Aspergillus niger and Candida albicans;
Introduction of 2-furyl moiety (9i) led to a 2-fold increase against the three test Gram-positive strains Staphylococcus aureus, Staphylococcus epidermidis and Micrococcus spp.;
Introduction of 2-thienyl group (9j) resulted in a decrease in activity by 4-fold against Staphylococcus aureus, and 2-fold against Staphylococcus epidermidis and Micrococcus spp.;
Shifting from phenyl group to 2-pyridyl group (9k) increased the antibacterial activity by 2-fold against Micrococcus spp., while retaining the same activity against Staphylococcus aureus and Staphylococcus epidermidis.
Figure 6.
Structure activity relationship for the newly synthesized compounds 9a–k concerning the antibacterial activity.
Collectively, it could be concluded that the presence of unsubstituted phenyl group (9a), presence of one donating methoxy group (9b), or the presence of the strongly deactivation NO2 group (9f), is the optimum for antibacterial activity. On the other hand, it could be concluded that the presence of bulky naphthyl group (9h) is the optimum for antifungal activity. Replacement of naphthyl group with any other group resulted in either decreasing or abolishing the antifungal activity.
2.2.2. The Antitumor Activity of the Tested Compounds
Cell Viability Assay
Firstly, to determine the cell viability, HPDE cell lines were treated with all new synthesized compounds 9a–k for 96 h using sulforhodamine B (SRB) assay. All newly synthesized compounds were proven non-toxic with IC50 more than 50 mg/mL (Table 2).
Table 2.
IC50 values of synthesized compounds in different cancer cell lines. Data are presented as mean ± SD (n = 3).
| Compounds | IC50 (µg/mL) | |||
|---|---|---|---|---|
| HepG-2 | Panc-1 | BxPC-3 | HPDE | |
| 9a | 10.96 ± 2.39 | 11.95 ± 0.76 | 14.39 ± 0.73 | >50 mg/mL |
| 9b | 8.53 ± 1.10 | 13.63 ± 1.16 | 14.88 ± 4.19 | >50 mg/mL |
| 9c | 19.08 ± 3.23 | 15.13 ± 4.13 | 21.47 ± 1.08 | >50 mg/mL |
| 9d | 22.71 ± 2.30 | 25.32 ± 1.91 | 18.05 ± 3.56 | >50 mg/mL |
| 9e | 15.04 ± 2.89 | 12.15 ± 1.29 | 13.28 ± 1.57 | >50 mg/mL |
| 9f | 6.39 ± 1.25 | 12.60 ± 1.20 | 14.18 ± 1.87 | >50 mg/mL |
| 9g | 32.53 ± 1.45 | 29.26 ± 4.23 | 25.70 ± 4.89 | >50 mg/mL |
| 9h | 17.34 ± 0.96 | 14.72 ± 2.87 | 12.60 ± 0.62 | >50 mg/mL |
| 9i | 27.017 ± 5.32 | 16.13 ± 1.820 | 21.83 ± 2.98 | >50 mg/mL |
| 9j | 25.54 ± 0.144 | 16.04 ± 3.18 | 21.42 ± 2.15 | >50 mg/mL |
| 9k | 33.11 ± 5.12 | 19.878 ± 3.35 | 7.27 ± 1.49 | >50 mg/mL |
| Doxorubicin | 5.11 ± 0.98 | 6.90 ± 0.93 | 7.31 ± 1.12 | >50 mg/mL |
Tested Compounds Suppress Cellular Proliferation of Cancer Cell Lines
The tested compounds’ effects on cellular proliferation of different cancer cell lines BxPC-3, Panc-1, HepG-2, and the normal immortalized cell line HPDE have been evaluated. SRB colorimetric assays have been conducted to evaluate cellular proliferation (Table 2). The tested compounds have varied anticancer activity ranging from moderate to very weak activity. Among all, compounds 9b and 9f displayed good anticancer activity against HepG2 cancer cell line with IC50 values = 8.53 and 6.39 µg/mL, respectively. Additionally, compound 9k exhibited good anticancer activity against PC3 cancer cell line with IC50 value = 7.27 µg/mL, in contrast with the normal HPDE cells that were the least affected after treatment (IC50 > 50 µg/mL). The remaining new synthesized compounds showed weak or very weak anticancer activity against the three cancer cell lines used.
Tested Compounds Can Induce Apoptotic Cell Death
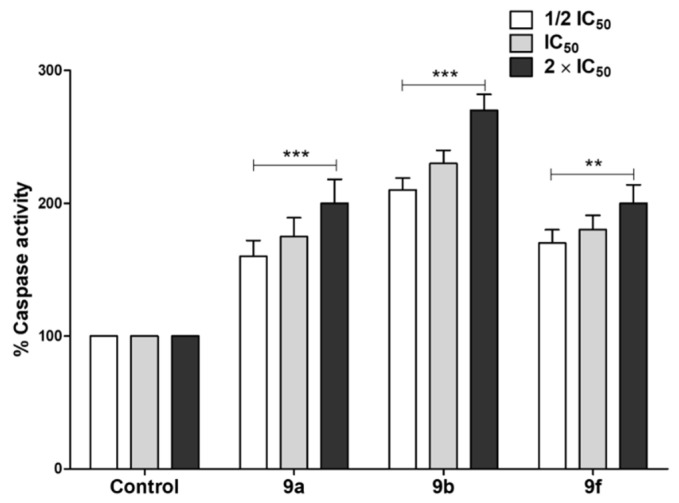
Apoptotic cascade induction is a main chemotherapy-induced cell death procedure [60]. The apoptotic effects of selected compounds 9a, 9b and 9f on pancreatic resistant cell lines and Panc-1 were assessed by quantification of caspase 3/7 levels (Figure 7). Our findings revealed that tested compounds triggered apoptosis through increasing the amounts of activated caspases 3/7 in Panc-1cell line compared with untreated controls. The apoptotic effect was dose-dependent, and the experiment was conducted in triplicate. The significance was analyzed using two-way ANOVA (Graphpad Prism 8 software) to compare the caspase levels at different concentrations. The results were significant statistically where p values < 0.05.
Figure 7.
Apoptotic effect of the active synthesized compounds. Quantification of caspase 3/7 levels was used to assess the apoptotic activities of tested compounds on cancer cells. The tested compounds, particularly 9b, significantly triggered the cancer cells’ apoptosis by increasing the dose. The experiment was conducted in triplicate and the date are shown as mean ± error values. (*** = p < 0.001, ** = p < 0.01).
2.3. Docking Study into Pseudomonas aeruginosa QS Receptors
The process in which bacterial populations are controlled is called quorum sensing (QS) in which cells communicate with each other using signaling molecules called autoinducers that are produced by bacterial cells and detected by receptors on other bacterial cells. The QS signaling system orchestrates numerous physiological functions in both Gram-positive and Gram-negative bacteria [51,61]. Targeting bacterial virulence is a promising approach to decreasing the development of bacterial resistance [51,54]. In this approach, we used synthesized compounds at their sub-MIC which did not affect the bacterial growth and hence will not increase the possibility of resistance development [47,53]. In this context, it was necessary to evaluate the ability of tested compounds to antagonize the QS, which is the key regulator of bacterial virulence.
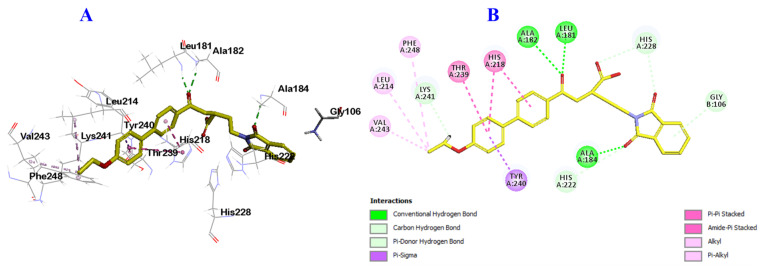
To explore the binding interactions and the capability of the most potent derivatives 9a, 9b and 9f to antagonize the QS receptors, the interactions between 9a, 9b, 9f and QS proteins were evaluated. Escherichia coli QS receptor was retrieved from the protein data bank (PDB: 1ROS) and molecular docking was carried out [62]. First, validation of the docking protocol was conducted by redocking of the ligand into the active site of the Pseudomonas aeruginosa QS receptor (PDB: 1ROS) (Figure 8). The RMSD value was less than 2 (0.835) which confirmed the validity of the docking results.
Figure 8.
Docking and binding mode of the ligand; 2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethyl-4-(4-ethoxy [1,1-biphenyl]-4-yl)-4-oxobutanoic acid (yellow) into the active site active site of the Escherichia coli QS protein (PDB: 1ROS). (A) 3D structure of the ligand (yellow), (B) 2D structure of the ligand (yellow).
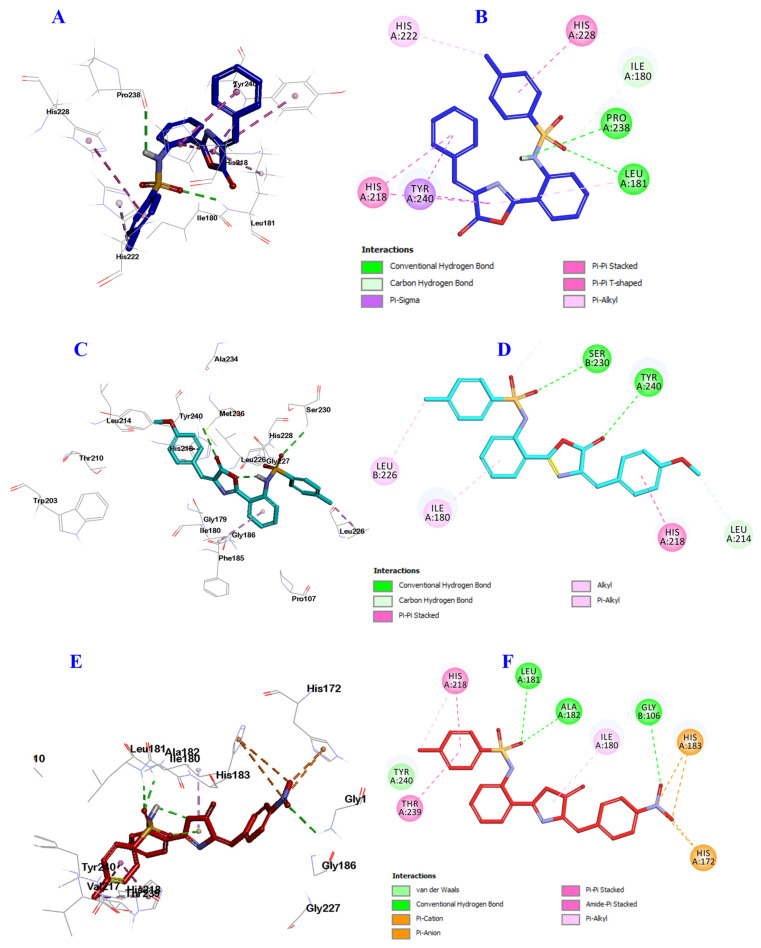
The results, as illustrated in Figure 8, displayed that derivatives 9a, 9b and 9f were well-accommodated inside the binding cavity of the receptor. From the docking results, compound 9a was incorporated into the formation of two hydrogen bonds, the oxygen of the sulfonamide group with Leu181, and the proton of sulfonamide nitrogen group with Pro238 amino acids. Additionally, it formed many hydrophobic interactions with Ile180, Leu181, His218, His222, His228 and Tyr240 amino acid residues (Figure 9A,B).
Figure 9.
Docking and binding mode of 9a (blue), 9b (cyan) and 9f (red) into the active site of the Escherichia coli QS protein (PDB: 1ROS). (A) 3D structure of 9a (blue), (B) 2D structure of 9a (blue), (C) 3D structure of 9b (cyan), (D) 2D structure of 9b (cyan) (E) 3D structure of 9f (red), and (F) 2D structure of 9f (red).
Similarly, compound 9b engaged in the formation of two hydrogen bonds but with different amino acids, the oxygen of sulfonamide group with Ser230, and the carbonyl oxygen of oxazolone ring with Tyr240. 9b formed many hydrophobic interactions with Ile180, Leu214, His218, Leu226 and His228 amino acid residues (Figure 9C,D).
Finally, compound 9f was involved in the formation of three hydrogen bonds; the oxygen of sulfonamide group incorporated in the formation of two hydrogen bonds with Gly106, and Leu181, while the oxygen of NO2 group engaged in the third hydrogen bond with Ala182. Additionally, compound 9f was involved in many hydrophobic interactions with His172, Ile180, His183, His218, Thr239 and Tyr240 amino acid residues (Figure 9E,F). These results are almost in agreement with the biological evaluation and may explain the possible reasons for enhanced anti-QS activity of compounds 9a, 9b and 9f, suggesting these three compounds for further study as novel promising antibiofilm and antimicrobial candidates.
3. Materials and Methods
3.1. Chemistry
Melting points were determined with a Gallenkamp (London, UK) melting point apparatus and are uncorrected. IR spectra (KBr, cm−1) were recorded on Bruker Vector, 22FT-IR (Fourier Transform Infrared (FTIR), Ettlingen, Germany) spectrometer. Unless otherwise specified, proton (1H) and carbon (13C) NMR spectra were recorded at room temperature in base filtered (CD3)2SO on a spectrometer operating at 400 MHz for proton and 100 MHz and 300 MHz for proton and 75 MHz for carbon nuclei. The signal due to residual (CH3)2SO appearing at δ H 2.5 and the central resonance of the (CD3)2SO “multipet” appearing at δ C 39.0 were used to reference 1H and 13C NMR spectra, respectively. 1H NMR data are recorded as follows: chemical shift (δ) (multiplicity, coupling constant(s) J (Hz), relative integral) where multiplicity is defined as s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet or combinations of the above. Elemental analyses were determined using a manual elemental analyzer Heraeus (Germany) and an automatic elemental analyzer CHN Model 2400 Perkin Elmer (USA) at Microanalytical Center, Faculty of Science, Cairo University, Egypt. All the results of elemental analyses corresponded to the calculated values within experimental error. Progress of the reaction was monitored by thin-layer chromatography (TLC) using precoated TLC sheets with ultraviolet (UV) fluorescent silica gel (Merck 60F254, Merck, Darmstadt, Germany), and spots were visualized by iodine vapors or irradiation with UV light (254 nm). All chemicals were purchased from Sigma-Aldrich or Lancaster Synthesis Corporation (Welwyn Garden, UK). Intermediate 3 [63] was prepared according to reported procedure.
3.1.1. General Procedure for the Synthesis of 2-((4-Methylphenyl)sulfonamido)benzoic acid 3
Anthranilic acid 1 (0.10 mmol) was dissolved in 30 mL sodium hydroxide (2 N) in a 500 mL conical flask. The mixture was stirred vigorously with a mechanical stirrer until the solid was almost completely dissolved. 4-toluenesulfonyl chloride 2 (0.10 mmol) was added in five portions and stirred vigorously for a further 1 h. The crystallized 4-toluenesulfonyl anthranilic was left in the refrigerator overnight. The crystals were filtered on a Buchner funnel, washed with ice cold water and dried at 100 °C. The product was crystallized from ethanol [63].
3.1.2. General Procedure for the Synthesis of N-(2-(1H-Benzo[d][1,2,3]triazole -1-carbonyl) phenyl)-4-methylbenzenesulfonamide 5
Thionyl chloride (0.08 mL, 1 mmol) was added to a solution of 1H-benzotriazole 4 (0.48 g, 0.4 mmol) in DCM (10 mL) at room temperature, the reaction mixture was stirred for 20 min, then acid 3 (0.2 g, 1 mmol) was added to the reaction mixture, which was stirred for 3 h at 25 °C. The reaction was diluted with DCM (50 mL) then the organic layer was washed with saturated Na2CO3 (3 × 20 mL), H2O (2 × 20 mL) and brine (1 × 10 mL), then dried (sodium sulfate), and filtered. Hexane (50 mL) was added to the filtrate, the obtained solid was dried under reduced pressure to give compound 5, which was crystallized from ethanol.
Yellowish solid, yield (89%); m.p. 190–192 °C. 1H NMR (400 MHz, DMSO-d6) δ: 2.27 (s, 3H), 7.04 (d, J = 8.4 Hz, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.56–7.50 (m, 1H), 7.66 (t, J = 7.2 Hz, 1H), 7.78 (dd, J = 7.6, 1.6 Hz, 1H), 7.85 (t, J = 8.2 Hz, 1H), 8.29 (t, J = 8.2 Hz, 2H), 10.06 (s, 1H) ppm. 13C NMR (100 MHz, DMSO) δ: 20.9, 114.3, 119.9, 125.2, 125.5, 126.4, 126.6, 128.6, 129.4, 130.5, 131.3, 131.3, 132.62, 135.28, 136.15, 143.22, 145.35, 165.72 ppm. Anal. Calcd for C20H16N4O3S: C, 61.21; H, 4.11; N, 14.28. Found: C, 61.39; H, 4.17; N, 14.45.
3.1.3. General Procedure for the Synthesis of (2-((4-Methylphenyl)sulfonamido) benzoyl)glycine 7
To a solution of benzenesulfonamide derivative 5 (0.31 g, 1 mmol) in acetonitrile (5 mL), a solution of glycine 6 (0.11 g, 1.5 mmol) in acetonitrile/H2O (7/3 mL) and triethylamine (0.12 mL, 1 mmol) was added. The reaction mixture was stirred at 25 °C for 12 h then monitored by TLC. After completion of the reaction, 6 N HCl (1 mL) was added, the reaction mixture was concentrated under reduced pressure. The residue thus obtained was partitioned between H2O (20 mL) and ethyl acetate (20 mL), and the separated organic layer was washed with 4 N HCl (3 × 5 mL) and brine (10 mL), then dried (MgSO4), filtered and concentrated under reduced pressure to deliver acid 6. The product was crystallized from ethanol.
Yellow solid, yield (80%); m.p. 198–200 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.31 (s, 3H), 3.91 (d, J = 6 Hz, 2H), 7.10 (t, J = 7.2 Hz, 1H), 7.34 (t, J = 8.4 Hz, 2H), 7.43–7.57 (m, 2H), 7.63–7.74 (m, 2H), 7.89 (d, J = 7.5 Hz, 1H), 9.13 (s, 1H), 11.48 (s, 1H), 12.74 (s, 1H) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 41.2, 116.5, 118.3, 119.1, 126.8, 128.4, 129.8, 132.7, 135.8, 138.5, 139.9, 143.7, 144.0, 168.6, 170.7 ppm. Anal. Calcd for C16H16N2O5S: C, 55.16; H, 4.63; N, 8.04. Found; C, 54.89; H, 4.67; N, 7.93.
3.1.4. General Procedure for the Synthesis of 9a–j
A mixture of (2-((4-methylphenyl)sulfonamido)benzoyl)glycine 7 (0.30 g, 1.10 mmol) and the appropriate aldehydes 8a–j (1.00 mmol) in acetic anhydride (1 mL) and fused sodium acetate (0.1 g, 1.2 mmol) was heated in an oil bath at 80 °C for 2 h. After cooling down at room temperature the mixture was allowed to stand for 24 h at 0 °C. The precipitate was filtered off and washed three times with ice-cooled ethanol (10 mL). The product was crystallized from ethanol.
3.1.5. (E)-N-(2-(4-Benzylidene-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9a
Whitish solid, yield (87%); m.p. 214–216 °C. 1H NMR (300 MHz, DMSO-d6) δ 2.32 (s, 3H, CH3), 7.21–7.25 (m, 2H, ArH), 7.35 (d, J = Hz, 2H, ArH), 7.48 (s, 1H, ArH), 7.48–7.46 (m, 6H, ArH), 7.78 (d, J = Hz, 2H, ArH), 7.88 (d, J = Hz, 1H, CH=C), 11.57 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ 20.9, 111.1, 117.5, 123.5, 126.9, 128.4, 129.1, 129.9, 130.0, 130.9, 131.1, 131.7, 132.0, 132.9, 134.8, 135.6, 139.0, 144.3, 162.8, 164.9 ppm. Anal. Calcd for C23H18N2O4S: C, 66.02; H, 4.34; N, 6.69. Found; C, 65.99; H, 4.28; N, 6.92.
3.1.6. (E)-N-(2-(4-(4-Methoxybenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9b
Straw yellow solid, yield (88%); m.p. 190–192 °C.1H NMR (300 MHz, DMSO-d6) δ: 2.32 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 7.09 (d, J = 9 Hz, 2H, ArH), 7.18–7.28 (m, 1H, ArH), 7.36 (d, J = 8.1 Hz, 2H, ArH), 7.46 (s, 1H, ArH), 7.61 (d, J = 7.6 Hz, 2H, ArH), 7.77 (d, J = 8.1 Hz, 2H, ArH), 7.86 (d, J = 7.8 Hz, 1H, CH=C), 8.23 (d, J = 9.0 Hz, 2H, ArH), 11.58 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 56.0, 106.8, 117.5, 120.8, 121.3, 121.9, 123.3, 123.6, 126.7, 126.9, 127.1, 129.7, 130.0, 130.1, 130.3, 130.6, 135.3, 139.0, 144.4, 150.4, 161.8, 173.1 ppm. Anal. Calcd for C24H20N2O5S: C, 64.27; H, 4.50; N, 6.25. Found: C, 64.53; H, 4.53; N, 6.34.
3.1.7. (E)-N-(2-(4-(2,5-diMethoxybenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9c
White solid, yield (85%); m.p. 220–222 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.32 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 7.13–7.25 (m, 2H, ArH), 7.26–7.34 (m, 3H, ArH), 7.61 (s, 2H, ArH), 7.72 (d, J = 7.8 Hz, 2H, ArH), 7.85 (d, J = 7.8 Hz, 2H, ArH), 7.95 (d, J = 6.1 Hz, 1H, CH=C), 10.92 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 55.7, 56.4, 112.6, 113.1, 114.9, 118.9, 120.4, 120.5, 121.5, 124.0, 124.6, 126.9, 129.9, 130.0, 130.6, 134.5, 135.6, 138.3, 153.3, 153.6, 162.6, 165.3 ppm. Anal. Calcd for C25H22N2O6S: C, 62.75; H, 4.63; N, 5.85. Found; C, 62.89; H, 4.67; N, 5.93.
3.1.8. (E)-4-Methyl-N-(2-(5-oxo-4-(3,4,5-trimethoxybenzylidene)-4,5-dihydrooxazol-2-yl)phenyl)be-nzenesulfonamide 9d
Yellowish solid, yield (78%); m.p. 230–232 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.31 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.92 (s, 6H, 2OCH3), 7.15–7.35 (m, 3H, ArH), 7.41 (s, 1H, ArH), 7.49–7.68 (m, 6H, ArH), 7.86 (d, J = 7.8 Hz, 1H, CH=C), 10.84 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 55.9, 56.2, 60.3, 107.5, 109.9, 111.9, 116.4, 119.6, 124.3, 126.9, 128.2, 129.9, 132.1, 134.4, 135.3, 138.1, 144.2, 153.1, 162.8, 165.9 ppm. Anal. Calcd for C26H24N2O7S: C, 61.41; H, 4.76; N, 5.51. Found; C, 61.44; H, 5.02; N, 5.62.
3.1.9. (E)-N-(2-(4-(4-Chlorobenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9e
Yellow solid, yield (85%); m.p. 234–236 °C. 1H NMR (300 MHz, DMSO-d6) δ 2.33 (s, 3H, CH3), 7.24–7.38 (m, 3H, ArH), 7.57–7.69 (m, 3H, ArH), 7.79–7.94 (m, 3H, 2ArH + CH=C), 8.32 (d, J = 8.6 Hz, 2H, ArH), 8.45 (d, J = 7.8 Hz, 2H, ArH), 11.48 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ 20.9, 111.1, 117.6, 123.5, 127.0, 129.1, 129.4, 129.9, 130.1, 131.4, 131.8, 133.4, 134.9, 135.6, 136.2, 139.0, 144.3, 163.1 ppm. Anal. Calcd for C23H17ClN2O4S: C, 61.00; H, 3.78; N, 6.19. Found; C, 60.78; H, 3.67; N, 6.39.
3.1.10. (E)-4-Methyl-N-(2-(4-(4-nitrobenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)benzenesulfonamide 9f
Pale yellow solid, yield (88%); m.p. 250–252 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.31 (s, 3H, CH3), 7.26 (t, J = 7.8 Hz, 1H, ArH), 7.37 (d, J = 8.1 Hz, 2H, ArH), 7.53–7.72 (m, 3H, ArH), 7.80 (d, J = 7.8 Hz, 2H, ArH), 7.92 (d, J = 8.1 Hz, 1H, CH=C), 8.32 (d, J = 8.4 Hz, 2H, ArH), 8.45 (d, J = 8.7 Hz, 2H, ArH), 11.48 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 111.0, 117.7, 119.0, 123.8, 126.9, 127.2, 129.2, 129.8, 130.3, 130.6, 132.5, 133.2, 135.4, 135.5, 139.2, 140.2, 144.4, 148.0, 164.5, 165.5 ppm. Anal. Calcd for C23H17N3O6S: C, 59.61; H, 3.70; N, 9.07. Found; C, 59.83; H, 3.79; N, 9.18.
3.1.11. (E)-N-(2-(4-(4-(Benzyloxy)benzylidene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9g
Whitish solid, yield (88%); m.p. 235–237 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.32 (s, 3H, CH3), 5.25 (s, 2H, OCH2-) 7.18–7.37 (m, 3H, ArH), 7.35–7.50 (m, 8H, ArH), 7.61 (d, J = 6.8 Hz, 2H, ArH), 7.77 (d, J = 8.3 Hz, 2H, ArH), 7.87 (d, J = 7.8 Hz, 1H, CH=C), 8.23 (d, J = 8.8 Hz, 2H, ArH), 11.60 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO) δ: 168.62, 162.64, 161.75, 161.36, 160.13, 144.38, 138.69, 136.45, 135.58, 134.56, 134.37, 131.51, 130.12, 129.74, 128.50, 128.05, 127.85, 126.99, 125.94, 123.61, 115.60, 111.40, 69.60, 20.97 ppm. Anal. Calcd for C30H24N2O5S: C, 68.69; H, 4.61; N, 5.34. Found: C, 68.66; H, 4.64; N, 5.38.
3.1.12. (E)-4-Methyl-N-(2-(4-(naphthalen-1-ylmethylene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)benzenesulfonamide 9h
White solid, yield (76%); m.p. 226–228 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.31 (s, 3H, CH3), 7.25-7.36 (m, 3H, ArH), 7.62-7.75 (m, 7H, ArH), 7.76-8.42 (m, 3H, ArH), 8.45 (d, J = 7.8 Hz, 2H, ArH), 8.75 (d, J = 7.5 Hz, 1H, CH=C), 11.60 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 21.00, 118.87, 119.5, 123.3, 127.0, 127.2, 128.8, 129.1, 129.9, 131.5, 132.3, 132.4, 133.3, 134.0, 135.9, 138.8, 143.9, 165.9, 168.3 ppm. Anal. Calcd for C27H20N2O4S: C, 69.22; H, 4.30; N, 5.98. Found; C, 68.98; H, 4.07; N, 5.92.
3.1.13. (E)-N-(2-(4-(Furan-2-ylmethylene)-5-oxo-4,5-dihydrooxazol-2-yl)phenyl)-4-methylbenzenesulfonamide 9i
Yellow solid, yield (84%); m.p. 191–193 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.33 (s, 3H, CH3), 7.08–7.12 (m, 2H, ArH), 7.12-7.32 (m, 3H, ArH), 7.36–7.47 (m, 3H, ArH), 7.51–7.88 (m, 3H, ArH), 7.90 (d, J = 8.4 Hz, 1H, CH=C) ppm, exchangeable 1H due to NH. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 116.8, 118.2, 118.8, 122.9, 123.1, 126.7, 126.8, 128.9, 129.8, 129.9, 131.5, 132.8, 134.3, 135.8, 139.2, 140.0, 143.7, 144.0, 169.7 ppm. Anal. Calcd for C21H16N2O5S: C, 61.76; H, 3.95; N, 6.86. Found; C, 61.89; H, 3.67; N, 6.93.
3.1.14. (E)-4-Methyl-N-(2-(5-oxo-4-(thiophen-2-ylmethylene)-4,5-dihydrooxazol-2-yl)phenyl)benzenesulfonamide 9j
Yellow solid, yield (90%); m.p. 196–198 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.32 (s, 3H, CH3), 7.24–7.35 (m, 1H, ArH), 7.38–7.50 (m, 2H, ArH), 7.58–7.63 (m, 1H, ArH), 7.77–7.87 (m, 4H, ArH), 7.90–8.22 (m, 3H, ArH), 8.24 (d, J = 7.6 Hz, 1H, CH=C), 11.49 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 111.1, 117.7, 123.5, 125.3, 126.9, 128.0, 128.7, 129.8, 130.0, 134.6, 135.6, 136.2, 136.8, 137.2, 138.6, 144.3, 161.3, 164.3 ppm. Anal. Calcd for C21H16N2O4S2: C, 59.42; H, 3.80; N, 6.60. Found; C, 59.61; H, 3.87; N, 6.82.
3.1.15. (E)-4-Methyl-N-(2-(5-oxo-4-(pyridin-4-ylmethylene)-4,5-dihydrooxazol-2-yl)phenyl)benzenesulfonamide 9k
Whitish solid, yield (78%); m.p. 210–212 °C. 1H NMR (300 MHz, DMSO-d6) δ: 2.32 (s, 3H, CH3), 7.21–7.38 (m, 5H, ArH), 7.59–8.19 (m, 7H, ArH), 8.20 (d, J = 7.8 Hz, 1H, CH=C), 11.27 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 20.9, 111. 7, 117.7, 123.5, 125.3, 126.9, 128.7, 129.8, 130.0, 134.5, 135.3, 135.8, 136.2, 137.0, 138.7, 144.0, 163.3, 165.7 ppm. Anal. Calcd for C22H17N3O4S; Calcd C, 63.00; H, 4.09; N, 10.02. Found: C, 63.31; H, 4.16; N, 10.21.
3.2. Biological Activity
3.2.1. Evaluation of Antimicrobial and Anti-Virulence Activities
Determination of Minimum Inhibitory Concentration (MIC)
The MICs of the synthesized compound were determined by agar dilution method according to the Clinical Laboratory and Standards Institute Guidelines (CLSI, 2015) [55,64]. Briefly, the tested strains were incubated overnight in tryptic soy broth (TSB) (Oxoid, United Kingdom) and then diluted in Muller–Hinton (MH) broth (Oxoid, United Kingdom) to turbidity approximating to the equivalent of 0.5 McFarland standard [65]. The suspensions were further diluted with sterile saline (1:10) and standardized inoculums (approximately 104 CFU per spot) were spotted on the surfaces of MH agar (Oxoid, United Kingdom) plates containing different concentrations of tested compounds and a control plate. The MICs were the lowest concentrations that inhibit growth on the plates after incubation at 37 °C for 20 h.
Excluding the Effect of Compounds on Bacterial Growth
To avoid any expected effect of tested compound on the bacterial virulence, the effect of compounds at their sub-MIC (½ MIC) on bacterial growth was evaluated [50,58]. The tested strains Pseudomonas aeruginosa ATCC 47,085 and Staphylococcus aureus ATCC 6538 were grown in Luria–Bertani (LB) Broth (Oxoid, Hampshire, United Kingdom) overnight at 37 °C in presence of tested compounds at sub-MIC (½ MIC). The experiment was conducted in triplicate, and the optical densities of bacterial growth were compared with control untreated bacteria. It is worth mentioning that the tested compounds were used at sub-MIC (½ MIC) in all the next performed tests to evaluate the anti-virulence activities.
Assay of Biofilm Formation
In order to evaluate the ability of the tested compounds to inhibit the biofilm formation, a strong biofilm forming P. aeruginosa ATCC 47,085 [47,66] and S. aureus ATCC 6538 [67] strains were used. As described earlier [68,69], suspensions of tested strains were prepared from overnight cultures in TSB and their optical densities were adjusted to OD600 of 0.4 (1×108 CFU/mL). Aliquots of 10 μL of the suspensions were added to 1 mL amounts of fresh TSB with or without sub-MICs of tested compounds. Then, 100 μL of TSB with or without tested compounds in sub-MIC were transferred into the wells of 96-well microtiter plates and incubated at 37 °C overnight. The non-adherent cells were removed, the wells were washed with sterile PBS, and left to dry. The attached biofilm forming cells were fixed with methanol for 25 min and stained with 1% crystal violet for 30 min. The excess dye was washed out and the crystal violet staining adhered biofilm forming cells were eluted by glacial acetic acid (33%). The experiment was conducted in triplicate and the absorbance was measured at 590 nm. The absorbances of tested strains treated with different compounds were expressed as mean ± standard error of percentage change from untreated tested strains control. The percentages of biofilm inhibition were calculated employing the following formula: (absorbance of control—absorbance in presence of tested compounds)/absorbance of control.
Assay of Protease Production
The effect of compounds 5, 7 and 10 on the production of protease was evaluated using casein substrate as described earlier [47,48]. Briefly, overnight cultures of P. aeruginosa and S. aureus were cultivated in LB broth in the presence or absence of compounds 5, 7 and 10 at ½ MIC for 24 h at 37 °C. The supernatants were collected, mixed (1:1) with 0.05 M casein in phosphate buffer (2%) and NaOH (0.1 M) at pH 7.0, and incubated for 15 min at 37 °C. The reaction was stopped by adding 2 mL of 0.4 M trichloroacetic acid for 30 min at 25 °C. Any precipitates were removed, and the optical densities were detected at 660 nm. The assays were performed in triplicate and the obtained optical densities of tested strains treated with compounds 5, 7 and 10 were expressed as mean ± standard error of percentage change from untreated tested strains control (positive control) and LB (negative control). The protease inhibition percentages were calculated: (O.D control—O.D tested compounds)/O.D control.
Assay of Hemolytic Activity
The anti-virulence effects of compounds 5, 7 and 10 on hemolytic activity of tested P. aeruginosa and S. aureus strains were assessed as described previously [52,53]. Optically adjusted bacterial cultures treated or untreated with tested compounds at sub-MIC were centrifuged, and 0.5 mL of supernatants were mixed with fresh 0.8 mL 2% erythrocyte (obtained from experimental animals) suspension in saline, and incubated for 2 h at 37 °C. A complete hemolysis positive control was prepared by addition of sodium dodecyl sulphate (SDS) to erythrocyte suspension, and negative control was prepared by incubation of erythrocytes in LB broth under the same conditions. After centrifugation, the absorbances of the lysed erythrocytes were measured at 540 nm by Biotek spectrofluorometer (Biotek, Winooski, VT, USA). The experiment was performed in triplicate, and the hemolysis of tested compound treated cultures were expressed as mean ± standard error of percentages compared with those obtained from untreated control cultures using the formula: (absorbance in presence or absence of tested compounds—absorbance of negative control)/(absorbance of positive control—absorbance of negative control).
Quantification of Staphyloxanthin Pigment
Staphyloxanthin and intermediate carotenoids were extracted from S. aureus treated or untreated with compounds 5, 7 and 10 at sub-MIC as described [59]. Bacterial cells were cultivated in TSB at 37 °C for 24 h, then cells were collected by centrifugation, and washed twice with phosphate-buffered saline (PBS). The obtained pellets were used to extract staphyloxanthin with methanol. The pellets (5 gm of) were resuspended in 20 mL methanol, and heated with gentle stirring at 55 °C in a water bath for 5 min. Then, the methanol extract liquids were cooled and centrifuged, and the absorbances of the produced staphyloxanthin were quantified spectrophotometrically at 450 nm (Biotek, Winooski, VT, USA). The experiment was repeated in triplicate, and the pigment absorbances in the presence of tested compounds were expressed as mean ± standard error of percentage change from untreated controls. The percentages of pigment production were calculated using the formula: (absorbance of control − absorbance in presence of tested compounds)/absorbance of control.
Quantification of Pyocyanin Pigment
The ability of selected compounds 5, 7 and 10 at sub-MIC to reduce the P. aeruginosa pyocyanin pigment production was estimated as described earlier [47,48]. P. aeruginosa overnight cultures were prepared and diluted in LB broth at 600 nm (O.D0.4), and 10 μL of the bacterial suspensions were added to 1mL broth tubes containing, or not, tested drugs at sub-MIC. After incubation for 48 h at 37 °C, the tubes were centrifuged and the pyocyanin in the supernatant was assayed spectrophotometrically at 691 nm by a Biotek spectrofluorometer (Biotek, Winooski, VT, USA). The experiment was repeated in triplicate, and the pyocyanin absorbances in the presence of tested compounds were expressed as mean ± standard error of percentage change from untreated controls. The percentages of pigment production were calculated using the formula: (absorbance of control − absorbance in presence of tested compounds)/absorbance of control.
3.2.2. Evaluation of Antitumor Activities of Synthesized Compounds
Effect of Synthesized Compounds on Cellular Proliferation
The pancreatic human cancer cell lines BxPC-3 and Panc-1, the human hepatocellular carcinoma (HepG-2), and the normal immortalized pancreatic cell line HPDE, that were used in this study, were obtained from the American Type Culture Collection (Rockville, USA). Cell lines were cultured and treated with the tested compounds or dimethyl sulfoxide (DMSO) as previously described [70,71]. Cells were cultivated in DMEM medium (Invitrogen, Carlsbad, CA, USA), supplemented with streptomycin, penicillin and fetal bovine serum (FCS) (Invitrogen, Carlsbad, CA, USA).
The sulforhodamine B (SRB) assay was employed to assess the anti-proliferative effects of tested compounds on cancer cells [60,72]. Cell lines were incubated and regularly treated with DMSO or increasing doses of tested compounds for 48 h. The cells were fixed with 10% trichloroacetic acid and stained with SRB fluorescent dye for 30 min. Then, the bounded SRB dye to cellular proteins was dissolved in 10 mM Tris base after washing excess dye with 1% acetic acid. The absorbance was measured at 510 nm in a reader (Biotek, Winooski, VT, USA).
Evaluation of Caspase-3/7 Activity
Caspases play critical roles in apoptosis. The apoptotic effects of selected compounds 5, 7 and 10 on pancreatic cell line Panc-1 were tested by quantification of caspase 3/7 using Caspase-Glo 3/7 assay kit (Promega, Fitchburg, MA, USA) as previously described [60,71]. Briefly, cells were treated with or without compounds 5, 7 and 10 (½ IC50, IC50, or 2 ×IC50) for 6 h. The prepared reagent Caspase-Glo 3/7 was added in equal volumes to cells, gently mixed, and incubated for 60 min at room temperature. The luminescence was measured, and the activity of caspase was presented as a percentage change from the untreated control.
3.3. In Silico Docking Study
Docking simulation study was carried out using Discovery Studio 2.5 software (Accelrys Inc., San Diego, CA, USA) [73]. For more details, see Supplementary Materials.
4. Conclusions
In summary, eleven oxazolone-benzenesulfonamide compounds 9a–k, were synthesized and characterized by IR, NMR (1H and 13C) and elemental analyses. The title compounds were evaluated for their in vitro antibiofilm, antimicrobial and anticancer activities. The majority of the tested compounds displayed potent antibacterial activity against both Gram-positive and -negative bacteria. Compounds 9a, 9b and 9f exhibited considerable antibacterial activity. Compound 9h exhibited the most potent antifungal activity. Compounds 9a, 9b, 9f and 9k showed good anticancer activity against different cancer cell lines. Importantly, several synthesized compounds showed a significant ability to inhibit the formation of biofilm by Pseudomonas aeruginosa and Staph. aureus. The compounds 9a, 9b and 9f displayed the most potent antibiofilm inhibition activity; that is why these three compounds were subjected to further investigation for their anti-virulence activities. The three compounds 9a, 9b and 9f significantly reduced the production of QS-controlled virulence factors. These findings are in great compliance with the ability to hinder the QS receptors in silico, indicating that these compounds can serve as anti-QS and anti-virulence agents.
Supplementary Materials
The following supporting information can be downloaded, 1H and 13C NMR Spectra for Compounds 9a–k.
Author Contributions
Conceptualization, A.J.A., A.M.M.A.-M. and T.S.I.; methodology, A.M.M.A.-M., E.S.T., M.Y. and W.A.H.H.; software, M.F.A.M. and A.J.A.; validation, A.J.A., T.S.I., E.S.T. and M.F.A.M.; formal analysis, M.Y. and W.A.H.H.; investigation, T.S.I. and M.F.A.M.; resources, A.M.M.A.-M. and A.J.A.; data curation, A.J.A., E.S.T., M.Y. and W.A.H.H.; writing—original draft preparation, A.J.A., T.S.I., E.S.T., M.Y. and W.A.H.H.; writing—review and editing, M.F.A.M. and W.A.H.H.; visualization, M.Y. and W.A.H.H.; supervision, A.M.M.A.-M. and A.J.A.; project administration, A.M.M.A.-M.; funding acquisition, A.J.A. and T.S.I. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, has funded this project under grant No. (RG-17-166-42). The authors, therefore, gratefully acknowledge DSR technical and financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ventola C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T Peer-Rev. J. Formul. Manag. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim T.S., Almalki A.J., Moustafa A.H., Allam R.M., Abuo-Rahma G.E.-D.A., El Subbagh H.I., Mohamed M.F.A. Novel 1,2,4-oxadiazole-chalcone/oxime hybrids as potential antibacterial DNA gyrase inhibitors: Design, synthesis, ADMET prediction and molecular docking study. Bioorg. Chem. 2021;111:104885. doi: 10.1016/j.bioorg.2021.104885. [DOI] [PubMed] [Google Scholar]
- 3.Hofny H.A., Mohamed M.F.A., Gomaa H.A.M., Abdel-Aziz S.A., Youssif B.G.M., El-koussi N.A., Aboraia A.S. Design, synthesis, and antibacterial evaluation of new quinoline-1,3,4-oxadiazole and quinoline-1,2,4-triazole hybrids as potential inhibitors of DNA gyrase and topoisomerase IV. Bioorg. Chem. 2021;112:104920. doi: 10.1016/j.bioorg.2021.104920. [DOI] [PubMed] [Google Scholar]
- 4.Sharma D., Misba L., Khan A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019;8:1–10. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uruén C., Chopo-Escuin G., Tommassen J., Mainar-Jaime R.C., Arenas J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics. 2021;10:3. doi: 10.3390/antibiotics10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins P., Jesus J., Santos S., Raposo L.R., Roma-Rodrigues C., Baptista P.V., Fernandes A.R. Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Tool Box. Molecules. 2015;20:16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eftekhari-Sis B., Zirak M., Akbari A. Arylglyoxals in Synthesis of Heterocyclic Compounds. Chem. Rev. 2013;113:2958–3043. doi: 10.1021/cr300176g. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y., Fang G., Chen H., Deng X., Tang Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021;226:113837. doi: 10.1016/j.ejmech.2021.113837. [DOI] [PubMed] [Google Scholar]
- 9.Fisk J.S., Mosey R.A., Tepe J.J. The diverse chemistry of oxazol-5-(4H)-ones. Chem. Soc. Rev. 2007;36:1432–1440. doi: 10.1039/b511113g. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues C.A.B., Martinho J.M.G., Afonso C.A.M. Synthesis of a Biologically Active Oxazol-5-(4H)-one via an Erlenmeyer–Plöchl Reaction. J. Chem. Educ. 2015;92:1543–1546. doi: 10.1021/ed500212t. [DOI] [Google Scholar]
- 11.Tandon M., Coffen D.L., Gallant P., Keith D., Ashwell M.A. Potent and selective inhibitors of bacterial methionyl tRNA synthetase derived from an oxazolone–dipeptide scaffold. Bioorg. Med. Chem. Lett. 2004;14:1909–1911. doi: 10.1016/j.bmcl.2004.01.094. [DOI] [PubMed] [Google Scholar]
- 12.Mesaik M.A., Rahat S., Khan K.M., Zia U., Choudhary M.I., Murad S., Ismail Z., Attaur R., Ahmad A. Synthesis and immunomodulatory properties of selected oxazolone derivatives. Bioorg. Med. Chem. 2004;12:2049–2057. doi: 10.1016/j.bmc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Hegazy W.A.H., Henaway M. Hepatitis C virus pathogenesis: Serum IL-33 level indicates liver damage. Afr. J. Microbiol. Res. 2015;9:1386–1393. doi: 10.5897/AJMR2015.7496. [DOI] [Google Scholar]
- 14.Mariappan G., Saha B.P., Datta S., Kumar D., Haldar P.K. Design, synthesis and antidiabetic evaluation of oxazolone derivatives. J. Chem. Sci. 2011;123:335–341. doi: 10.1007/s12039-011-0079-2. [DOI] [Google Scholar]
- 15.Pinto I.L., West A., Debouck C.M., DiLella A.G., Gorniak J.G., O’Donnell K.C., O’Shannessy D.J., Patel A., Jarvest R.L. Novel, selective mechanism-based inhibitors of the herpes proteases. Bioorg. Med. Chem. Lett. 1996;6:2467–2472. doi: 10.1016/0960-894X(96)00456-8. [DOI] [Google Scholar]
- 16.Taile V., Hatzade K., Gaidhane P., Ingle V. Synthesis and Biological Activity of 4-(4-Hydroxybenzylidene)-2-(substituted styryl) oxazol-5-ones and Their o-glucosides. Turk. J. Chem. 2009;33:295–305. [Google Scholar]
- 17.Jat L., Mishra R., Pathak D. Synthesis and anticancer activity of 4-Benzylidene-2-phenyloxazol-5 (4H)-one derivatives. Int. J. Pharm. Pharm. Sci. 2012;4:378–380. [Google Scholar]
- 18.Hassanein H.H., Khalifa M.M., El-Samaloty O.N., El-Rahim M.A., Taha R.A., Magda, Ismail M.F. Synthesis and biological evaluation of novel imidazolone derivatives as potential COX-2 inhibitors. Arch. Pharmacal. Res. 2008;31:562. doi: 10.1007/s12272-001-1193-6. [DOI] [PubMed] [Google Scholar]
- 19.Witvrouw M., Pannecouque C., De Clercq E., Fernández-Alvarez E., Marco J.L. Inhibition of Human Immunodeficiency Virus Type (HIV-1) Replication by some Diversely Functionalized Spirocyclopropyl Derivatives. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 1999;332:163–166. doi: 10.1002/(SICI)1521-4184(19995)332:5<163::AID-ARDP163>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Tsukumo Y., Harada D., Manabe H. Pharmacological Characterization of Itch-Associated Response Induced by Repeated Application of Oxazolone in Mice. J. Pharmacol. Sci. 2010;113:255–262. doi: 10.1254/jphs.10050FP. [DOI] [PubMed] [Google Scholar]
- 21.Khan K.M., Mughal U.R., Khan M.T.H., Zia U., Perveen S., Iqbal Choudhary M. Oxazolones: New tyrosinase inhibitors; synthesis and their structure–activity relationships. Bioorg. Med. Chem. 2006;14:6027–6033. doi: 10.1016/j.bmc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Hamidian H., Azizi S. Synthesis of novel compounds containing morpholine and 5(4H)-oxazolone rings as potent tyrosinase inhibitors. Bioorg. Med. Chem. 2015;23:7089–7094. doi: 10.1016/j.bmc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Kiptoo P.K., Paudel K.S., Hammell D.C., Hamad M.O., Crooks P.A., Stinchcomb A.L. In vivo evaluation of a transdermal codrug of 6-β-naltrexol linked to hydroxybupropion in hairless guinea pigs. Eur. J. Pharm. Sci. 2008;33:371–379. doi: 10.1016/j.ejps.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandel R., Mammen D. Synthesis and Study of Some Compounds Containing Oxazolone Ring, Showing Biological Activity. Indian J. Chem. 2008;47:932–937. doi: 10.1002/chin.200839111. [DOI] [Google Scholar]
- 25.Sánchez C., Méndez C., Salas J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006;23:1007–1045. doi: 10.1039/B601930G. [DOI] [PubMed] [Google Scholar]
- 26.Rix U., Zheng J., Remsing Rix L.L., Greenwell L., Yang K., Rohr J. The dynamic structure of jadomycin B and the amino acid incorporation step of its biosynthesis. J. Am. Chem. Soc. 2004;126:4496–4497. doi: 10.1021/ja031724o. [DOI] [PubMed] [Google Scholar]
- 27.Wookey A., Turner P.J., Greenhalgh J.M., Eastwood M., Clarke J., Sefton C. AZD2563, a novel oxazolidinone: Definition of antibacterial spectrum, assessment of bactericidal potential and the impact of miscellaneous factors on activity in vitro. Clin. Microbiol. Infect. 2004;10:247–254. doi: 10.1111/j.1198-743X.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 28.Goodman M., Levine L. Peptide Synthesis via Active Esters. IV. Racemization and Ring-Opening Reactions of Opitcally Active Oxazolones. J. Am. Chem. Soc. 1964;86:2918–2922. doi: 10.1021/ja01068a030. [DOI] [Google Scholar]
- 29.Ghorab M.M., Alsaid M.S., El-Gaby M.S.A., Safwat N.A., Elaasser M.M., Soliman A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016;124:299–310. doi: 10.1016/j.ejmech.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 30.Alaoui S., Dufies M., Driowya M., Demange L., Bougrin K., Robert G., Auberger P., Pagès G., Benhida R. Synthesis and anti-cancer activities of new sulfonamides 4-substituted-triazolyl nucleosides. Bioorg. Med. Chem. Lett. 2017;27:1989–1992. doi: 10.1016/j.bmcl.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Durgun M., Turkmen H., Zengin G., Zengin H., Koyunsever M., Koyuncu I. Synthesis, characterization, in vitro cytotoxicity and antimicrobial investigation and evaluation of physicochemical properties of novel 4-(2-methylacetamide)benzenesulfonamide derivatives. Bioorg. Chem. 2017;70:163–172. doi: 10.1016/j.bioorg.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Dai H.-X., Stepan A.F., Plummer M.S., Zhang Y.-H., Yu J.-Q. Divergent C–H Functionalizations Directed by Sulfonamide Pharmacophores: Late-Stage Diversification as a Tool for Drug Discovery. J. Am. Chem. Soc. 2011;133:7222–7228. doi: 10.1021/ja201708f. [DOI] [PubMed] [Google Scholar]
- 33.Gul H.I., Tugrak M., Sakagami H., Taslimi P., Gulcin I., Supuran C.T. Synthesis and bioactivity studies on new 4-(3-(4-Substitutedphenyl)-3a,4-dihydro-3H-indeno[1,2-c]pyrazol-2-yl) benzenesulfonamides. J. Enzym. Inhib. Med. Chem. 2016;31:1619–1624. doi: 10.3109/14756366.2016.1160077. [DOI] [PubMed] [Google Scholar]
- 34.Lal J., Gupta S.K., Thavaselvam D., Agarwal D.D. Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Eur. J. Med. Chem. 2013;64:579–588. doi: 10.1016/j.ejmech.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Lu X.-Y., Wang Z.-C., Ren S.-Z., Shen F.-Q., Man R.-J., Zhu H.-L. Coumarin sulfonamides derivatives as potent and selective COX-2 inhibitors with efficacy in suppressing cancer proliferation and metastasis. Bioorg. Med. Chem. Lett. 2016;26:3491–3498. doi: 10.1016/j.bmcl.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Supuran C.T., Casini A., Scozzafava A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 2003;23:535–558. doi: 10.1002/med.10047. [DOI] [PubMed] [Google Scholar]
- 37.Scozzafava A., Owa T., Mastrolorenzo A., Supuran C.T. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003;10:925–953. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 38.Ning X., Guo Y., Ma X., Zhu R., Tian C., Zhang Z., Wang X., Ma Z., Liu J. Design, synthesis and pharmacological evaluation of (E)-3,4-dihydroxy styryl sulfonamides derivatives as multifunctional neuroprotective agents against oxidative and inflammatory injury. Bioorg. Med. Chem. 2013;21:5589–5597. doi: 10.1016/j.bmc.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Qin H.-L., Zhang Z.-W., Lekkala R., Alsulami H., Rakesh K.P. Chalcone hybrids as privileged scaffolds in antimalarial drug discovery: A key review. Eur. J. Med. Chem. 2020;193:112215. doi: 10.1016/j.ejmech.2020.112215. [DOI] [PubMed] [Google Scholar]
- 40.Ho T.C.S., Chan A.H.Y., Ganesan A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem. 2020;63:12460–12484. doi: 10.1021/acs.jmedchem.0c00830. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C., Rakesh K.P., Ravidar L., Fang W.-Y., Qin H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019;162:679–734. doi: 10.1016/j.ejmech.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashkenazi A., Fairbrother W.J., Leverson J.D., Souers A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017;16:273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 43.Yap J.L., Chen L., Lanning M.E., Fletcher S. Expanding the Cancer Arsenal with Targeted Therapies: Disarmament of the Antiapoptotic Bcl-2 Proteins by Small Molecules. J. Med. Chem. 2017;60:821–838. doi: 10.1021/acs.jmedchem.5b01888. [DOI] [PubMed] [Google Scholar]
- 44.Ballatore C., Huryn D.M., Smith A.B., III Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem. 2013;8:385–395. doi: 10.1002/cmdc.201200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammazzalorso A., De Filippis B., Giampietro L., Amoroso R. N-acylsulfonamides: Synthetic routes and biological potential in medicinal chemistry. Chem. Biol. Drug Des. 2017;90:1094–1105. doi: 10.1111/cbdd.13043. [DOI] [PubMed] [Google Scholar]
- 46.Parsonnet J. Bacterial infection as a cause of cancer. Environ. Health Perspect. 1995;103((Suppl. S8)):263–268. doi: 10.1289/ehp.95103s8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aldawsari M.F., Khafagy E.S., Saqr A.A., Alalaiwe A., Abbas H.A., Shaldam M.A., Hegazy W.A.H., Goda R.M. Tackling Virulence of Pseudomonas aeruginosa by the Natural Furanone Sotolon. Antibiotics. 2021;10:871. doi: 10.3390/antibiotics10070871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hegazy W.A.H., Khayat M.T., Ibrahim T.S., Nassar M.S., Bakhrebah M.A., Abdulaal W.H., Alhakamy N.A., Bendary M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms. 2020;8:1285. doi: 10.3390/microorganisms8091285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saqr A.A., Aldawsari M.F., Khafagy E.S., Shaldam M.A., Hegazy W.A.H., Abbas H.A. A Novel Use of Allopurinol as A Quorum-Sensing Inhibitor in Pseudomonas aeruginosa. Antibiotics. 2021;10:1385. doi: 10.3390/antibiotics10111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbas H.A., Hegazy W.A.H. Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS ONE. 2020;15:e0231625. doi: 10.1371/journal.pone.0231625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegazy W.A.H., Rajab A.A.H., Abu Lila A.S., Abbas H.A. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World J. Diabetes. 2021;12:1832–1855. doi: 10.4239/wjd.v12.i11.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khayyat A.N., Abbas H.A., Khayat M.T., Shaldam M.A., Askoura M., Asfour H.Z., Khafagy E.S., Abu Lila A.S., Allam A.N., Hegazy W.A.H. Secnidazole Is a Promising Imidazole Mitigator of Serratia marcescens Virulence. Microorganisms. 2021;9:2333. doi: 10.3390/microorganisms9112333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khayyat A.N., Abbas H.A., Mohamed M.F.A., Asfour H.Z., Khayat M.T., Ibrahim T.S., Youns M., Khafagy E.-S., Abu Lila A.S., Safo M.K., et al. Not Only Antimicrobial: Metronidazole Mitigates the Virulence of Proteus mirabilis Isolated from Macerated Diabetic Foot Ulcer. Appl. Sci. 2021;11:6847. doi: 10.3390/app11156847. [DOI] [Google Scholar]
- 54.Khayyat A.N., Hegazy W.A.H., Shaldam M.A., Mosbah R., Almalki A.J., Ibrahim T.S., Khayat M.T., Khafagy E.S., Soliman W.E., Abbas H.A. Xylitol Inhibits Growth and Blocks Virulence in Serratia marcescens. Microorganisms. 2021;9:1083. doi: 10.3390/microorganisms9051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegazy W.A.H., Khayat M.T., Ibrahim T.S., Youns M., Mosbah R., Soliman W.E. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz. J. Microbiol. 2021;52:627–638. doi: 10.1007/s42770-021-00465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbas H.A., Hegazy W.A.H. Targeting the virulence factors of Serratia marcescens by ambroxol. Roum. Arch. Microbiol. Immunol. 2017;76:27–32. [Google Scholar]
- 57.Askoura M., Youns M., Hegazy W.A.H. Investigating the influence of iron on Campylobacter jejuni transcriptome in response to acid stress. Microb. Pathog. 2020;138:103777. doi: 10.1016/j.micpath.2019.103777. [DOI] [PubMed] [Google Scholar]
- 58.Askoura M., Hegazy W.A.H. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog. Dis. 2020;78:ftaa011. doi: 10.1093/femspd/ftaa011. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Suo Y., Zhang D., Jin F., Zhao H., Shi C. Genetic and Virulent Difference Between Pigmented and Non-pigmented Staphylococcus aureus. Front. Microbiol. 2018;9:598. doi: 10.3389/fmicb.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youns M., Askoura M., Abbas H.A., Attia G.H., Khayyat A.N., Goda R.M., Almalki A.J., Khafagy E.S., Hegazy W.A.H. Celastrol Modulates Multiple Signaling Pathways to Inhibit Proliferation of Pancreatic Cancer via DDIT3 and ATF3 Up-Regulation and RRM2 and MCM4 Down-Regulation. Onco Targets. 2021;14:3849–3860. doi: 10.2147/OTT.S313933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bendary M.M., Ibrahim D., Mosbah R.A., Mosallam F., Hegazy W.A.H., Awad N.F.S., Alshareef W.A., Alomar S.Y., Zaitone S.A., Abd El-Hamid M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics. 2020;10:25. doi: 10.3390/antibiotics10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morales R., Perrier S., Florent J.-M., Beltra J., Dufour S., De Mendez I., Manceau P., Tertre A., Moreau F., Compere D., et al. Crystal Structures of Novel Non-peptidic, Non-zinc Chelating Inhibitors Bound to MMP-12. J. Mol. Biol. 2004;341:1063–1076. doi: 10.1016/j.jmb.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 63.Kamal A., Reddy J.S., Bharathi E.V., Dastagiri D. Base-free monosulfonylation of amines using tosyl or mesyl chloride in water. Tetrahedron Lett. 2008;49:348–353. doi: 10.1016/j.tetlet.2007.11.044. [DOI] [Google Scholar]
- 64.Vishwa B., Moin A., Gowda D.V., Rizvi S.M.D., Hegazy W.A.H., Abu Lila A.S., Khafagy E.S., Allam A.N. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics. 2021;13:79. doi: 10.3390/pharmaceutics13010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agha K.A., Abo-Dya N.E., Ibrahim T.S., Abdel-Aal E.H., Hegazy W.A.H. Benzotriazole-Mediated Synthesis and Antibacterial Activity of Novel N-Acylcephalexins. Sci. Pharm. 2016;84:484–496. doi: 10.3390/scipharm84030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegazy W.A.H., Abbas H.A. Evaluation of the role of SsaV ‘Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr. J. Biotechnol. 2017;16:718–726. doi: 10.5897/AJB2016.15852. [DOI] [Google Scholar]
- 67.Berlutti F., Frioni A., Natalizi T., Pantanella F., Valenti P. Influence of sub-inhibitory antibiotics and flow condition on Staphylococcus aureus ATCC 6538 biofilm development and biofilm growth rate: BioTimer assay as a study model. J. Antibiot. 2014;67:763–769. doi: 10.1038/ja.2014.66. [DOI] [PubMed] [Google Scholar]
- 68.El-Hamid A., Marwa I., Y El-Naenaeey E.S., Hegazy W.A.H., Mosbah R.A., Nassar M.S., Bakhrebah M.A., Abdulaal W.H., Alhakamy N.A., Bendary M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics. 2020;9:667. doi: 10.3390/antibiotics9100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Askoura M., Almalki A.J., Lila A.S.A., Almansour K., Alshammari F., Khafagy E.-S., Ibrahim T.S., Hegazy W.A.H. Alteration of Salmonella enterica Virulence and Host Pathogenesis through Targeting sdiA by Using the CRISPR-Cas9 System. Microorganisms. 2021;9:2564. doi: 10.3390/microorganisms9122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aldawsari M.F., Alalaiwe A., Khafagy E.S., Al Saqr A., Alshahrani S.M., Alsulays B.B., Alshehri S., Abu Lila A.S., Danish Rizvi S.M., Hegazy W.A.H. Efficacy of SPG-ODN 1826 Nanovehicles in Inducing M1 Phenotype through TLR-9 Activation in Murine Alveolar J774A.1 Cells: Plausible Nano-Immunotherapy for Lung Carcinoma. Int. J. Mol. Sci. 2021;22:6833. doi: 10.3390/ijms22136833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Youns M., Hegazy W.A.H. The Natural Flavonoid Fisetin Inhibits Cellular Proliferation of Hepatic, Colorectal, and Pancreatic Cancer Cells through Modulation of Multiple Signaling Pathways. PLoS ONE. 2017;12:e0169335. doi: 10.1371/journal.pone.0169335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al Saqr A., Khafagy E.S., Alalaiwe A., Aldawsari M.F., Alshahrani S.M., Anwer M.K., Khan S., Lila A.S.A., Arab H.H., Hegazy W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials. 2021;11:808. doi: 10.3390/nano11030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ibrahim T.S., Moustafa A.H., Almalki A.J., Allam R.M., Althagafi A., Md S., Mohamed M.F.A. Novel chalcone/aryl carboximidamide hybrids as potent anti-inflammatory via inhibition of prostaglandin E2 and inducible NO synthase activities: Design, synthesis, molecular docking studies and ADMET prediction. J. Enzym. Inhib. Med. Chem. 2021;36:1067–1078. doi: 10.1080/14756366.2021.1929201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.