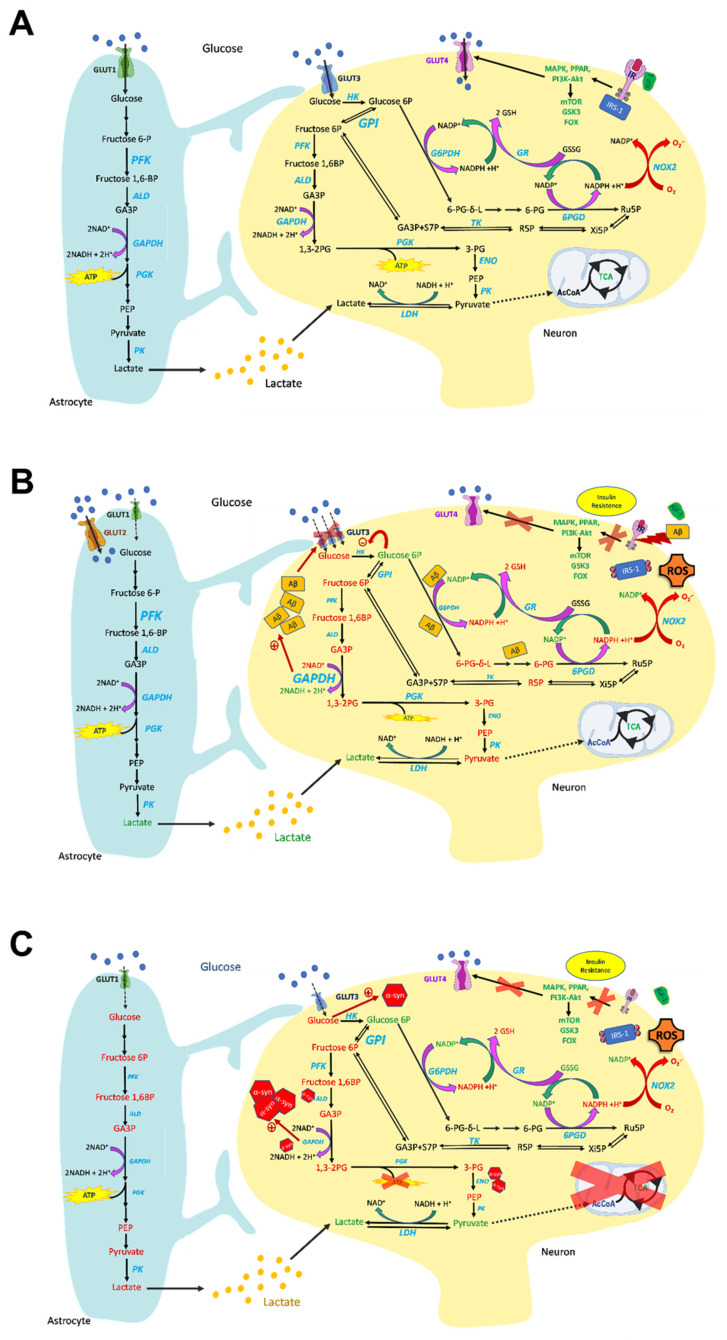
Figure 1.
Overview of glucose metabolism in normal (A), AD (B), and PD (C) brains. (A) Glucose is the main energy substrate in the brain, as it is the principal source of ATP. In the brain, glucose is taken up from the bloodstream by specific glucose transporters, to be metabolized through glycolysis. Pyruvate enters the TCA cycle coupled with OXPHOS and ATP synthesis. Glucose transport in the brain occurs via GLUT1 in astrocytes and GLUT3 in neurons. Insulin-dependent GLUT4 transport also occurs in the brain, and its activation leads to insulin receptor signaling, occurring through the PI3K/Akt and MAPK pathways, regulating the brain’s main cellular functions. Glucose metabolism is prominent in the brain—especially in astrocytes—and strongly interconnected among the different cell types. Glucose 6-phosphate (G6P) can be channeled into the pentose phosphate pathway (PPP) to support NADPH synthesis, which is necessary to sustain the brain’s antioxidant defense, and this is enhanced by the recycling of fructose 6-phosphate (F6P) originating from the PPP back to G6P due to high GPI activity. (B) Glucose metabolism is impaired in AD brains. Signs of impaired insulin signaling cascade are present in AD brains, with insulin resistance and downregulation of insulin receptors, which contribute to brain glucose hypometabolism. In AD, decreased glucose metabolism impacts the metabolic crosstalk between astrocytes and neurons, as the lactate shuttle is impaired, leading to reduced ATP synthesis. GLUT1 and -3 are decreased in AD brains, and this correlates to glucose hypometabolism, and is a major pathological sign of AD, whereas GLUT2 increases, indicating prominent astroglial activation in AD brains. Glycolysis increases in astrocytes and microglia, and this is associated with neurodegeneration. G6P and fructose 1,6-bisphosphate (F1,6BP) levels are inversely correlated with age. Hexokinase (HK), PK, and PFK are downregulated in neurons, whereas GAPDH is upregulated and can promote Aβ amyloidogenesis. Aβ plays a role in impairing the PPP, leading to G6P accumulation—which inhibits HK activity—and to decreased defense against ROS. Aβ oligomers also reduce the IR and promote synaptic spine deterioration. (C) Glucose metabolism is dysfunctional in PD brains, and this mirrors a significant loss of IR. Furthermore, IRS phosphorylation deactivates insulin signaling, leading to insulin resistance. Moreover, the glucose transporters GLUT1 and GLUT3 are downregulated. The decrease in glucose metabolism, prominent both in neurons and in astrocytes, is associated with pyruvate and lactate accumulation and deleterious ATP depletion. Depletion of the ATP-generating enzyme PGK is associated with neuronal deficits with PD-like symptoms. Low glucose promotes α-synuclein (α-syn) aggregation. α-Syn fibrils interact with GAPDH, aldolase (ALD), and enolase (ENO), and their activities are consequently decreased. Furthermore, GAPDH directly regulates α-syn aggregation and apoptotic neuronal cell death. Increased metabolite levels are reported in green, whereas decreased levels are reported in red.