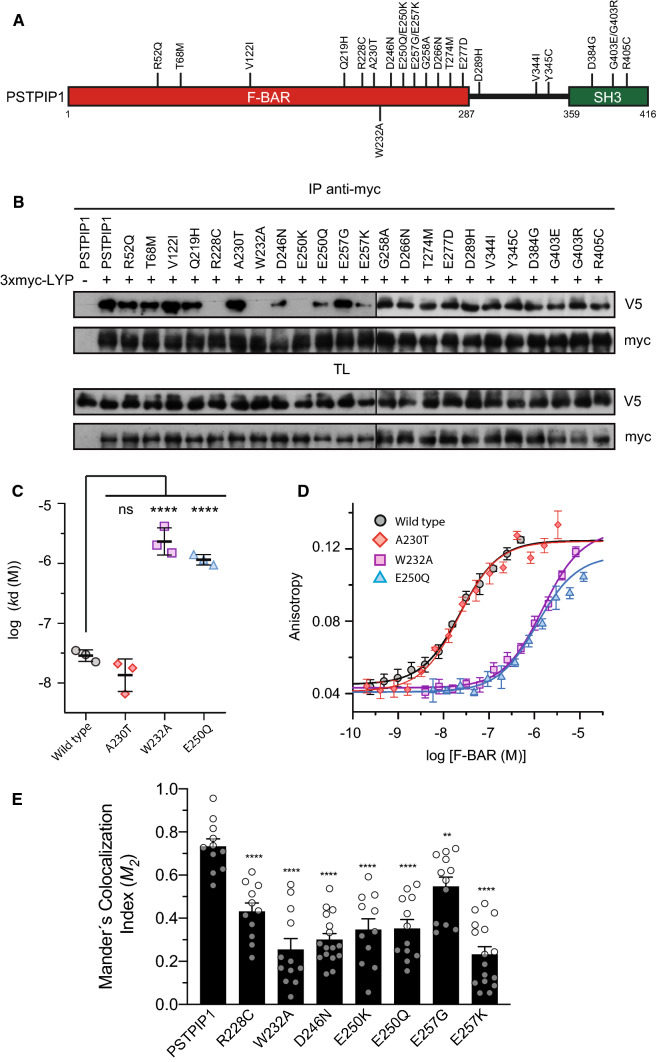
Fig. 1.
Interaction of LYP with of PSTPIP1 mutants associated to immune diseases. A Domain structure of PSTPIP1 with the mutations studied on this work. Mutations described in patients suffering from autoinflammatory diseases and CVID are indicated on top, while W232A mutation that is not related to disease is on the lower part. B HEK293 cells were transfected with PSTPIP1-V5 wild type and several mutants associated with autoinflammatory diseases, as indicated in the top of the panels, and with 3xmyc-LYP. Lysates were immunoprecipitated with myc antibody (Ab) and PSTPIP1 bound to LYP was detected by IB with Ab for the V5 epitope. Expression of the proteins was verified by IB in total lysates (TL) with the same Abs. C Dissociation constants (kd) of the interaction of the F-BAR domain of PSTPIP1, wild type and indicated mutants, with fluorescein-LYP-CTH. Data are means ± standard deviation (SD), n = 3 independent experiments, one shown in D. Statistical comparison to the kd of the wild type F-BAR was analyzed using ANOVA followed by Dunnett’s test. ****p < 0.0001. D Representative binding isotherms of the F-BAR domain of PSTPIP1, wild type and mutants, to fluorescein-labeled LYP-CTH (2 nM), measured by fluorescence anisotropy. Data points are means ± SD of measurement replicates; lines represent the fitted binding curves. E HEK293 cells co-transfected with LYP-mCherry (red) and wild type or indicated mutants of PSTPIP1-eGFP (green). Manders’ colocalization indexes (M2) were calculated. Error bars represent SEM (n > 10). **p < 0.01; ****p < 0.0001, by Student’s t test