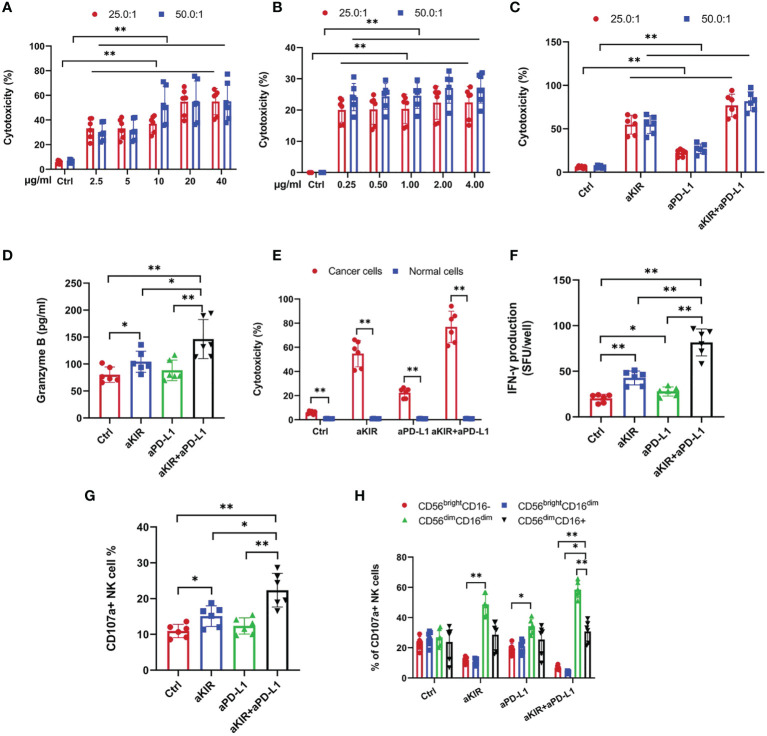
Figure 1.
Lirilumab and Avelumab Promote NK Cell Disinhibition and Cytotoxicity against HPV+ Cervical Cancer Cells in vitro. Blood-derived NK cells and autologous biopsy-derived malignant squamous cells obtained from HPV+ cervical cancer patients (n = 24) were incubated with DMSO (control), lirilumab (aKIR, 20 µg/ml) and/or avelumab (aPD-L1, 2.0 µg/ml), respectively, for 24 hours and then cultured together for 30 min. (A-C) NK cell-mediated lysis of cancer cells as measured by a target-based, europium-release cytotoxicity assay at E:T ratios of 25:1 and 50:1. (D) Blood-derived NK cells and autologous biopsy-derived malignant squamous cells or normal cervical squamous cells were incubated with control, aKIR, and/or aPD-L1 for 24 hours and then cultured together for 30 min for NK cell-mediated cytotoxicity assays. (E, F) NK cell (E) granzyme B and (F) IFN-γ production as measured by the ELISPOT effector-cell-based assays. (G) Percentages of CD107a+ NK cells. (H) Percentages of CD56dimCD16dim and CD56dimCD16+ CD107a+ NK cells. *P < 0.05, **P < 0.01 [(A–C) two-way ANOVA, (A, B) mAb concentration factor × E:T ratio factor, (C) mAb factor × E:T ratio factor, (D, F–H) one-way ANOVA, or (E) Student’s t-test]. n=3 biological replicates×3 technical replicates.