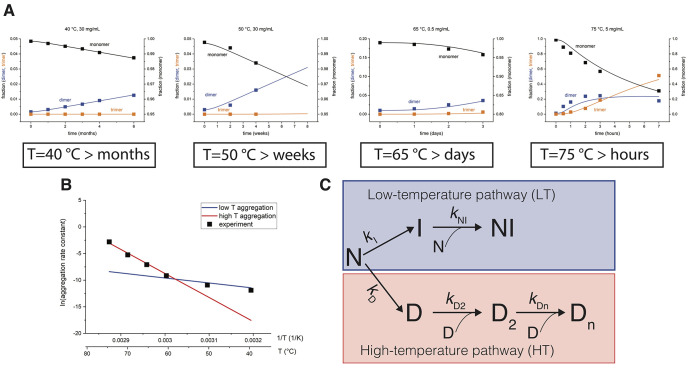
Figure 1.
Branched kinetic model describes antibody aggregation in a broad range of temperatures. (A) Aggregation time course of mAb1 measured at different temperatures and concentrations in 20 mM histidine buffer, pH 6.0. Fractions of monomers and oligomers (dimers and trimers) were determined from the size exclusion chromatography (SEC) chromatograms (see Figure S1) at different time points. Solid lines show the global fit to the data using a branched kinetic mechanism. The corresponding kinetic parameters are reported in Table S1. (B) Arrhenius plot for mAb1 aggregation shows a biphasic behavior. The observed curvature in the Arrhenius plot can be explained by using two competitive kinetic pathways [low-temperature (LT) and high-temperature (HT) aggregation pathway] with different temperature dependencies (red and blue lines). The temperature determines the aggregation flux through either pathway. (C) Branched aggregation mechanism describes mAb aggregation in a broad range of temperatures. In both LT and HT pathways, the first step involves the conversion of a native monomer N to an intermediate (I or D), followed by a relatively faster formation of oligomers NI or D2 and Dn.