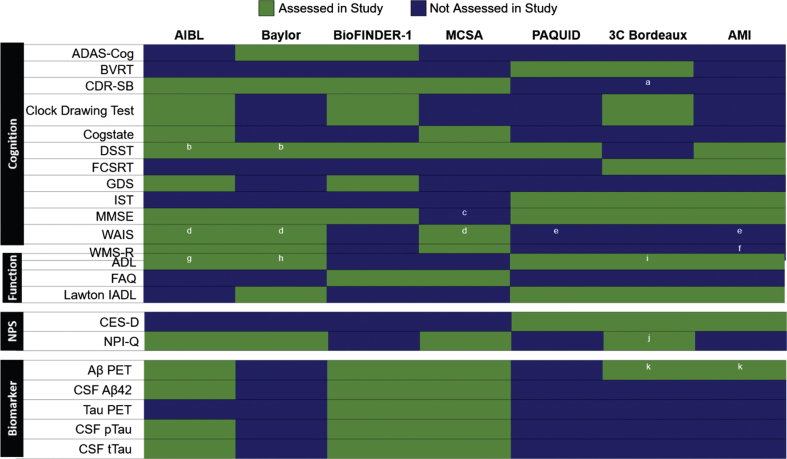
Fig. 1.
Assessments conducted in at least two of the CONCORD-AD cohorts. Includes assessments performed in cohort studies in different geographic regions. Aβ, amyloid-β; AD, Alzheimer’s disease; ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive subscale; ADL, Activities of daily living; AIBL, Australian Imaging, Biomarkers & Lifestyle Flagship Study of Ageing; AMI, AGRICA-MSA-Institut fédératif de recherche en santé publique/Aging Multidisciplinary Investigation; BioFINDER-1, Biomarkers For Identifying Neurodegenerative Disorders Early and Reliably; BVRT, Benton Visual Retention test; CDR-SB, Clinical Dementia Rating–Sum of Boxes; CES-D, Centre for Epidemiologic Studies Depression; CSF, cerebrospinal fluid; CU, cognitively unimpaired; DSST, Digit Symbol Substitution Test; FAQ, Functional Assessment Questionnaire; FCSRT, Free and Cued Selective Reminding Test; GDS, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; IST, Isaacs Set Test; MCI, mild cognitive impairment; MCSA, Mayo Clinic Study of Aging; MINI, Mini-International Neuropsychiatric Interview; MMSE, Mini-Mental State Examination; ND, non-demented; NPI-Q, Neuropsychiatric Inventory Questionnaire; NPS, neuropsychiatric symptoms; PET, positron emission tomography; pTau, phosphorylated tau; tTau, total tau; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition; WAIS-R, Wechsler Adult Intelligence Scale–Revised; WMS-R, Wechsler Memory Scale–Revised; 3C Bordeaux, Three-City Study. aUsed in the COGICARE sub-study of 3C Bordeaux in all participants with dementia; bDSST can be derived from the WAIS-R used in Baylor and from WAIS-III in AIBL; cMMSE score can be derived from the Short Test of Mental Status used in MCSA; dWAIS-R in Baylor, WAIS-III in AIBL; eWechsler similarities test used; fWechsler story memory test; gADL Inventory; hLawton and Brody instrumental ADL Scales; iKatz scale; jShort form used in the COGICARE sub-study of 3C; kAmyloid-PET available only on a subsample at the follow-up.