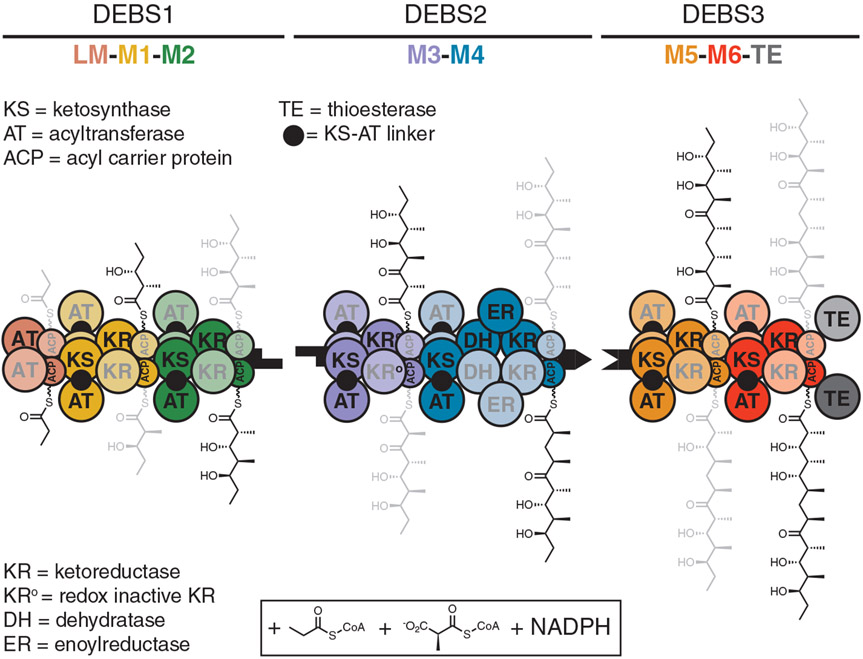
Fig. 1. Model for channeling of biosynthetic intermediates by the DEBS assembly line.
DEBS includes three homodimeric polypeptides (DEBS 1 to 3) with a collective molecular mass of 2.1 MDa and harbors six elongation modules (M1 to M6) flanked by a loading module (LM) and a terminal TE domain. When provided propionyl-CoA (starter unit), (2S)-methylmalonyl-CoA (extender units), and NADPH, the assembly line synthesizes 6-deoxyerythronolide B, the macrocyclic aglycone of the antibiotic erythromycin. Two growing polyketide chains are synthesized in parallel by two sets of active sites of the homodimeric DEBS; individual monomeric copies of each module are distinguished by heavy and light shading. Earlier experiments established that translocation of the polyketide intermediate from one module to the next involves intrapolypeptide ACP→KS channeling, whereas polyketide chain elongation involves interpolypeptide KS→CP channeling across the dimer interface (5). The cryo-EM structures solved in this work provide a clear mechanistic rationale for this observation. Shape-complementary black tabs at the end of each protein represent docking domains that facilitate intermodular polyketide translocation (40).