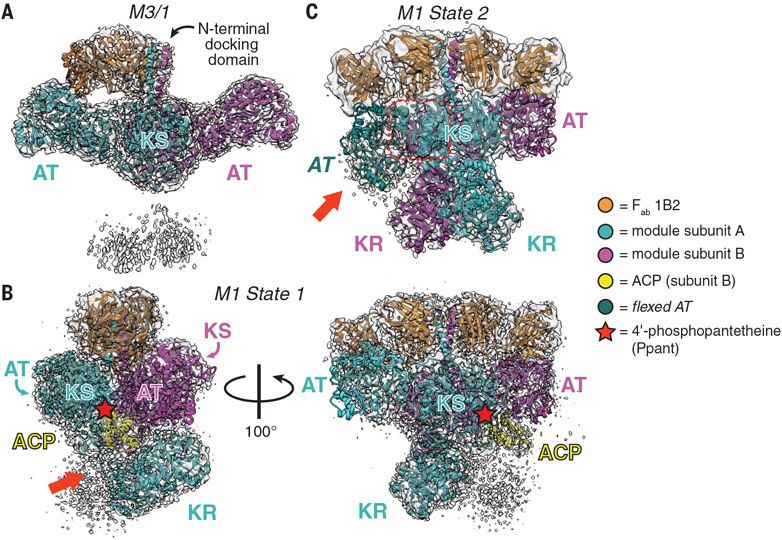
Fig. 2. Snapshots of three asymmetric cryo-EM structures.
(A) The 3.2-Å-resolution structure of a hybrid module composed of domains from DEBS M3/1 (map threshold = 0.28). (B and C) Two distinct states of DEBS M1. (B) The 3.2-Å-resolution structure of State 1 in which the P-pant cofactor (red star) of one ACP domain is bound in the KS active site of the other monomer (map threshold = 0.53). The red arrow highlights the resolved AT-KR linker (figs. S12, S13, and S14A and supplementary text). (C) The 4.1-Å-resolution structure of State 2 featuring two symmetric KR domains in addition to an atypically flexed AT domain (red arrow). Dashed red box is the KS-AT linker that is not conformationally altered by AT flexing (fig. S17 and movie S1; map threshold = 0.35).Domain acronyms are defined in Fig. 1. Note that the color scheme (bottom) adopted for this figure is different from that of Fig. 1 because a different color palette was needed to clearly distinguish the module subunits, Fabs, and ACP.