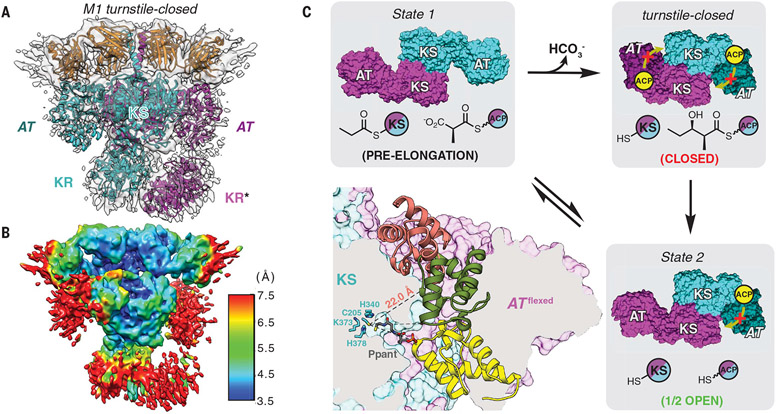
Fig. 4. Cryo-EM analysis of DEBS M1 in its turnstile-closed state.
(A) The 4.3-Å-resolution cryo-EM structure of M1 in its post-elongation (turnstile-closed) state was solved, as described in the text (map threshold = 0.26). *The KR domain of subunit B (magenta) has been modeled here, although it is absent from the deposited coordinates because of orientation ambiguity and reduced local resolution [~7.5 Å in (B); PDB 7M7J]. (B) Resolution map for the structure in (A) (map threshold = 0.26). (C) Bottom left panel, ACP inaccessibility of the KS active site in this state. Accessibility of the cleft resulting from AT flexing was evaluated using the KS-ACP interaction pose observed in State 1 (yellow ACP) as well as two prior models for KS-ACP interaction during intermodular polyketide translocation (6, 7), (29, 30) (salmon and green ACPs, respectively). Although the yellow and green poses are disfavored for steric reasons, the P-pant arm of the salmon pose is insufficiently long to access the KS active site (see fig. S23 for details). Remaining panels show a model for asymmetric turnstile closing of M1. Upon chain elongation, the pre-elongation state (State 1) undergoes a conformational change into its turnstile-closed state (A) to occlude the KS active site from an upstream or intramodular ACP, shown in yellow (movie S3). The duration of the turnstile-closed state is long enough to allow the ACP-bound diketide to undergo KR-catalyzed reduction and translocation to the downstream module, after which one catalytic subunit of the module returns to its translocation-competent state (State 2). Note that only the KS and AT catalytic domains are shown for clarity, and the KS and ACP acylation states are depicted for both modular subunits behaving equivalently but asynchronously.