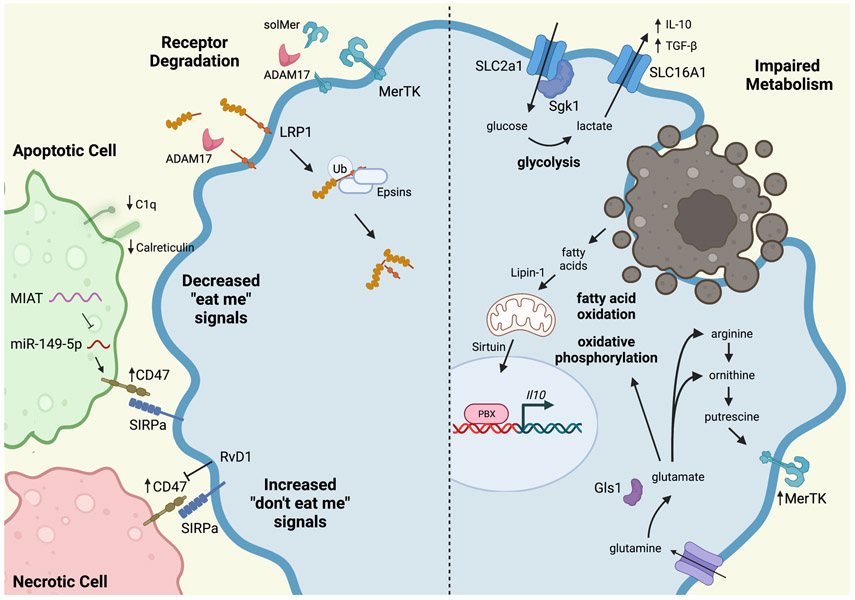
Figure 3. Mechanisms of Defective Efferocytosis in Atherosclerosis.
A variety of impairments contribute to defective efferocytosis in atherosclerosis. Efferocytosis receptors, including MerTK and LRP1, can be cleaved by ADAM17. LRP can also be ubiquitinated and degraded by Epsins. Apoptotic cells express decreased levels of “eat me” signals, such as C1q and calreticulin. Apoptotic and necrotic cells inappropriately express the “don’t eat me” signal, CD47, that prevents their efficient uptake by macrophages. This can be overcome by treatment with resolvin D1 (RvD1). Metabolism also plays an important role in efficient efferocytosis, particular in continual efferocytosis where successive apoptotic cells must be consumed. Glycolysis produces lactate, which increases IL-10 production and enhances subsequent efferocytosis. Digestion of apoptotic cells provides fatty acids as a substrate for fatty acid oxidation and oxidative phosphorylation, which also increase production of IL-10. Apoptotic cell-derived arginine and ornithine are converted to putrescine, which leads to enhanced, persistent MerTK expression on the macrophage, further enhancing efferocytosis. Glutamine is converted to glutamate, which contributes to oxidative phosphorylation, but also produces arginine and ornithine to feed the polyamine synthesis pathway. (Illustration Credit: Ben Smith).