Abstract
Otitis media (OM) is a leading cause of pediatric antibiotic use. Introduction of the 13-valent pneumococcal conjugate vaccine (PCV13) led to reductions in OM among US children, though its impact on OM-related antibiotic use remains unclear. Among 499 683 Tennessee children <2 years of age, the OM-related antibiotic fill rate was stable after PCV13 introduction.
Keywords: otitis media, antibiotic use, pneumococcal conjugate vaccines
Otitis media (OM) is a major driver of healthcare utilization, healthcare costs, and antibiotic use among children [1–5]. Antibiotic use for OM treatment is common and contributes to the development and spread of antibiotic-resistant bacteria [6], such that reducing OM-related antibiotic use is a public health priority.
Streptococcus pneumoniae is a leading bacterial cause of OM. The introduction of 7-valent pneumococcal conjugate vaccine (PCV7) in the United States (US) in 2000 provided protection against 7 pneumococcal serotypes and led to a sustained reduction in invasive pneumococcal disease, pneumonia, and OM among young children [7–13]. In 2010, the 13-valent pneumococcal conjugate vaccine (PCV13) replaced PCV7 in the US and has been associated with additional declines in OM in the US and elsewhere [14]. However, the impact of PCV13 introduction on OM-related antibiotic use remains unclear. Thus, we compared the incidence of first OM-related antibiotic use before and after PCV13 introduction among young children <2 years in the US state of Tennessee.
METHODS
We identified a retrospective cohort of children <2 years enrolled in the Tennessee Medicaid (TennCare) program from July 2004 to June 2015. TennCare is the managed Medicaid program in Tennessee. The TennCare data, including enrollment information, healthcare utilization information, and pharmacy fill data was supplemented with linked birth certificate data and registry data for all state hospital-based encounters [15, 16]. We followed children from birth through the earliest of loss of enrollment, age 2 years, first OM episode, or death. The project was approved by the Vanderbilt and Tennessee Department of Health institutional review boards, and the Division of TennCare.
We compared the incidence of first OM-related antibiotic use in the period before PCV13 introduction (July 2004 to June 2009) to the period after PCV13 introduction (July 2010 to June 2015). We defined July 2009–June 2010 as a transition period due to PCV13 introduction in early 2010 and unusually high influenza activity in late 2009 (related to influenza A/H1N1/pdm09 circulation). We defined study periods from July to June to include only a single US annual viral respiratory season in each year. The outcome was the monthly rate of first OM-related antibiotic prescription fills. We identified first OM episodes using coded diagnoses in the outpatient setting (International Classification of Diseases, Ninth Revision, Clinical Modification: 381, 381.0∗–381.4∗, 382.∗). We defined an OM-related antibiotic prescription fill as an antibiotic prescription filled within 3 days of the child’s first observed OM diagnosis. For each calendar month in the study period, we calculated the rate of first OM-related antibiotic fills per 1000 person-years (PY) by dividing the total monthly number of children with a first OM-related antibiotic fill by the total PY in that month.
To account for secular trends in risk factors for OM and antibiotic prescribing, we identified the monthly prevalence of risk factors for OM in the population, including child characteristics (birth year and month, sex, race), delivery characteristics (ventilation required at birth, birthweight) and maternal characteristics (gestational hypertension, prenatal care, previous poor outcome, previous preterm birth, tobacco use in pregnancy). For binary risk factors, we calculated the monthly prevalence as the number of total days among patients with the risk factor divided by the total days of follow-up each month. For continuous measures, we calculated the daily median value in the cohort, and calculated the median of daily medians each month.
We used segmented linear regression with autoregressive errors to assess the impact of PCV13 on the trend of first OM-related antibiotic use. We compared the observed post-PCV13 trend (July 2010 to June 2015, 60 monthly observations) to the pre-PCV13 trend (July 2004 to June 2009, 60 monthly observations). Furthermore, we compared observed monthly rates in the post-PCV13 period to projected rates assuming no PCV13 introduction. We visually examined correlograms and residuals and accounted for first and second order autocorrelation. We accounted for seasonality by including a calendar month indicator in the regression model. We included covariates in the model only if they were associated with the outcome (P < .2). We conducted a secondary analysis to determine the monthly proportion of all first OM episodes with an associated antibiotic fill (first OM episode with related antibiotic fill / first OM episodes) to examine whether antibiotic prescribing patterns changed over time. Analyses were done using Stata, version 17.1 (StataCorp LP).
RESULTS
The retrospective cohort encompassed 499 683 children <2 years of age who contributed 539 077 PY of observation (Supplementary Table 1). We did not observe changes in the prevalence of any risk factor in the cohort that temporally aligned with PCV13 introduction in 2010 (and none were associated with the outcome in the model accounting for autocorrelation and seasonality).
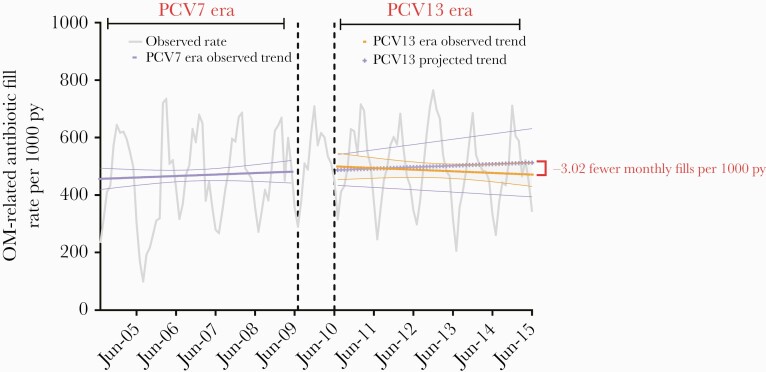
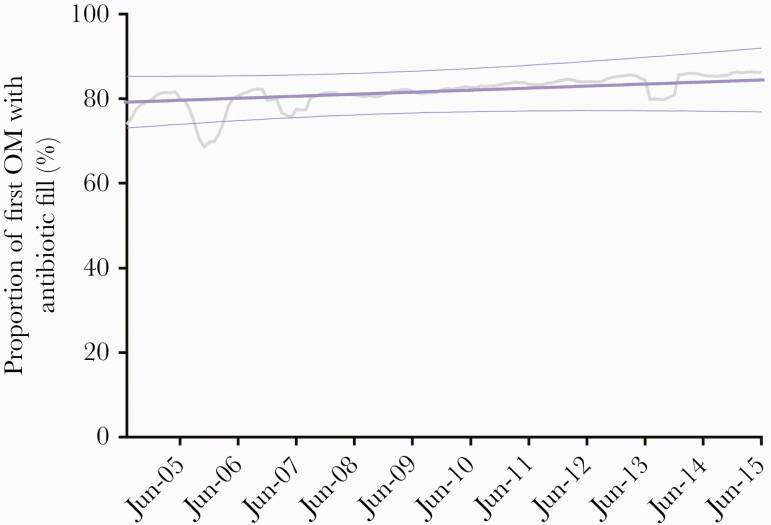
We observed 494.3 monthly OM-related antibiotic fills per 1000 PY in the post-PCV13 period compared to 458.6 during the pre-PCV13 period, though a nonsignificant trend decline was observed between periods (trend difference, –3.02 [95% confidence interval {CI}, –8.74 to 2.70]) (Figure 1). In a comparison of the final month of the post-PCV13 period (June 2015) to the final month of the pre-PCV13 period (June 2009), we observed no significant difference in the monthly rate (rate difference, –23.66 [95% CI, –148.42 to 101.11]). Similarly, the observed rate in June 2015 was lower, but not significantly, than the predicted rate in June 2015 assuming no PCV13 introduction (rate difference, –93.51 [95% CI, –369.02 to 182.00]). We observed a significantly increasing trend in the proportion of first episodes of OM treated with antibiotics during the study period (coefficient, 6.8% [95% CI, 1.9%–11.8%]), with no observed impact related to PCV13 introduction (Figure 2).
Figure 1.
Monthly rates and trend of first otitis media–related antibiotic fills per 1000 person-years among children aged <2 years before and after 13-valent pneumococcal conjugate vaccine introduction in Tennessee (2004–2015). Abbreviations: OM, otitis media; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PY, person-years.
Figure 2.
Monthly moving average (6 months, gray line) and trend (purple line) of the proportion of first episodes of otitis media with an antibiotic prescription in children aged <2 years, Tennessee Medicaid, 2004–2015. Abbreviation: OM, otitis media.
DISCUSSION
In this study of children <2 years old in Tennessee, we observed that PCV13 introduction in 2010 did not significantly impact first OM–related antibiotic use. However, we did observe an increasing proportion of first OM episodes for which an antibiotic was used throughout the study period.
Prior clinical trials have demonstrated that PCV7 administration at an early age in children is associated with a substantial relative risk reduction of developing pneumococcal OM (estimates range from 7% to 37% reduction) [17]. Population-based studies have also demonstrated that PCV7 introduction into the childhood vaccination schedule was associated with reductions in emergency department (16%–41%) and outpatient (4%–19%) visits for OM in the US [18]. Subsequently, PCV13 introduction was associated with reductions in serotype-specific pneumococcal OM [19], with evidence suggesting that it is also associated with reductions in all-cause OM [20–24]. In a study using the same underlying retrospective cohort as the present study, PCV13 introduction was associated with a reduction in the risk of first, subsequent, and recurrent OM among children aged <2 years in Tennessee [24]. Similarly, in a population-based time-series analysis among 191 596 children in Sweden (1999–2013), the incidence of OM declined from 33 099 (95% CI, 32 559–33 648) to 25 710 (95% CI, 25 433–25 990) per 100 000 PY in the PCV13 era compared to the PCV7 era [20]. However, an individual-level survival analysis in the same population did not find a significantly lower individual risk of first OM among children aged <5 years in the PCV13 era compared to the PCV7 era [20].
The impact of PCV13 introduction on OM-related antibiotic use remains less clear. Among a representative sample of US physician encounters, PCV13 introduction was associated with a 25.1% reduction in the annual incidence rate of OM among children aged 0–9 years (2011: 284.1 per 1000; 2016: 212.7 per 1000) and specifically a 25.0% reduction among children <2 years of age. The projected cumulative reduction in OM-related antibiotic prescriptions was 9.2 million fewer prescriptions (95% CI, –4.4 million to –14.0 million) in 2012–2016 compared to 2011 and was attributed to the reduction of OM incidence rather than reduced prescribing for the treatment of OM [23]. Relatedly, in our current study, we report a relatively stable frequency of OM-related antibiotic fills among children <2 years of age, in spite of an increasing proportion of OM episodes with an affiliated antibiotic fill.
The observed increase in the proportion of OM episodes that involved an antibiotic prescription is noteworthy. Future studies should explore differences in antibiotic prescribing patterns for OM, as changes in antibiotic prescribing could have implications for the development and management of recurrent OM episodes and on the development and spread of antimicrobial resistance [25, 26]. One such study by Suaya et al reported minor increases in amoxicillin use (prescribed in 56% of OM episodes in 2016 compared to 51% in 2011) and minor reductions in azithromycin use in 2016 (4.6%) compared to 2011 (6.7%) among US children [23].
A strength of the study was the use of linked administrative data with state vital records and a hospitalization registry for complete ascertainment of OM episodes among a large retrospective cohort of children. The use of pharmacy fill data strengthened the validity of the measurement of OM-related antibiotic use compared to prescribing information or patient recall. The autoregressive modeling design allowed us to assess and account for the underlying trends of each outcome prior to PCV13 introduction and to account for autocorrelation and the seasonality of OM-related antibiotic fills. Another advantage of this ecological study design was the ability to capture both direct and indirect effects of PCV13 on the population.
A limitation of our study is the retrospective, ecological design that is subject to the possibility that secular trends in risk factors for OM or OM-related prescribing practices might have impacted OM-related antibiotic use independent of PCV13 introduction. Additionally, the design required the aggregation of patient-level information into monthly estimates, thus reducing the effective sample size to only 132 data points (11 years) and reducing the power of the study to detect small changes. Another limitation is that the use of coded diagnoses to identify OM episodes limited our ability to examine the severity of the disease, which could influence antibiotic prescribing decisions [27, 28]. Our study also lacked a control comparison as the potential impact of PCV13 introduction on other childhood respiratory diseases made it difficult to identify an appropriate control condition. Finally, the study was limited to children enrolled in a Medicaid program in a single US state, thus limiting the potential generalizability of our findings.
We observed that OM-related antibiotic use was very high in our study population and that use did not change significantly after PCV13 introduction. Importantly, our study did demonstrate an increasing trend in the use of antibiotics related to first episodes of OM during the study period.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Data availability. Data are not publicly available.
Patient consent statement. The design of the work was approved by the Tennessee Department of Health and Vanderbilt University Medical Center institutional review boards with a waiver of written patient consent as it involves previously collected administrative data.
Disclaimer. The funders did not have a role in any aspect of developing the report or in the decision to submit the article for publication.
Financial support. A. D. W. was supported by the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) program supported by the National Institutes of Health (NIH) (grant number 5K12HD043483) and by the National Institute on Drug Abuse (award number K01DA051683). C. G. G. was supported in part by Sanofi Pasteur, and the Vanderbilt Trans-Institutional Program Vanderbilt Study of Antimicrobial Resistance, and the National Institutes of Health ([NIH] NIAID K24AI148459).
Potential conflicts of interest. C. G. G. reports consulting fees from Pfizer and Merck, and research support from Sanofi-Pasteur and the Campbell Alliance/Syneos Health. A. D. W. reports consulting fees from the Tennessee Department of Health. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kaur R, Morris M, Pichichero ME.. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 2017; 140:e20170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tong S, Amand C, Kieffer A, Kyaw MH.. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008–2014. BMC Health Serv Res 2018; 18:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang SS, Johnson KM, Ray GT, et al. . Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29:3398–412. [DOI] [PubMed] [Google Scholar]
- 4. Hersh AL, Jackson MA, Hicks LA.. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013; 132:1146–54. [DOI] [PubMed] [Google Scholar]
- 5. Monasta L, Ronfani L, Marchetti F, et al. . Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 2012; 7:e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costelloe C, Metcalfe C, Lovering A, et al. . Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340:c2096. [DOI] [PubMed] [Google Scholar]
- 7. Pilishvili T, Lexau C, Farley MM, et al. . Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 8. Pelton S. Otitis media: re-evaluation of diagnosis and treatment in the era of antimicrobial resistance, pneumococcal conjugate vaccine, and evolving morbidity. Pediatr Clin North Am 2005; 3:711–28. [DOI] [PubMed] [Google Scholar]
- 9. Zhou F, Shefer A, Kong Y, Nuorti JP.. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997– 2004. Pediatrics 2008; 121:253–60. [DOI] [PubMed] [Google Scholar]
- 10. Poehling KA, Szilagyi P, Grijalva CG, et al. . Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics 2007; 119:707–15. [DOI] [PubMed] [Google Scholar]
- 11. Griffin MR, Zhu Y, Moore MR, et al. . U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grijalva CG, Poehling KA, Nuorti JP, et al. . National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 2006; 118:865–73. [DOI] [PubMed] [Google Scholar]
- 13. Grijalva CG, Nuorti JP, Arbogast PG, et al. . Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]
- 14. Lau WC, Murray M, El-Turki A, et al. . Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine 2015; 33:5072–6079. [DOI] [PubMed] [Google Scholar]
- 15. Griffin MR, Mitchel E, Moore MR, et al. . Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines—Tennessee, 1998-2012. MMWR Morb Mortal Wkly Rep 2014; 63:995–8. [PMC free article] [PubMed] [Google Scholar]
- 16. Grijalva CG, Nuorti JP, Zhu Y, Griffin MR.. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis 2010; 50:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Sévaux JLH, Venekamp RP, Lutje V, et al. . Pneumococcal conjugate vaccines for preventing acute otitis media in children. Cochrane Database Syst Rev 2020; 11:CD001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grijalva CG, Griffin MR.. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Rev Vaccines 2008; 7:83–95. [DOI] [PubMed] [Google Scholar]
- 19. Dagan R, van der Beek BA, Ben-Shimol S, et al. . Effectiveness of the seven- and thirteen valent pneumococcal conjugate vaccines against vaccine-serotype otitis media. Clin Infect Dis 2021; 73:650–8. [DOI] [PubMed] [Google Scholar]
- 20. Edmondson-Jones M, Dibbern T, Hultberg M, et al. . The effect of pneumococcal conjugate vaccines on otitis media from 2005 to 2013 in children aged ≤5 years: a retrospective cohort study in two Swedish regions. Hum Vacc Immunother 2021; 17:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grobman A, Reddy P, Wolfovitz A, et al. . The epidemiological and financial effects of pneumococcal vaccination on otitis media related admissions in the United States. Ann Otol Rhinol Laryngol 2021; 130:760–8. [DOI] [PubMed] [Google Scholar]
- 22. Chapman R, Sutton K, Dillon-Murphy D, et al. . Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: a modelling analysis. Vaccine 2020; 38:7138–45. [DOI] [PubMed] [Google Scholar]
- 23. Suaya JA, Gessner BD, Fung S, et al. . Acute otitis media, antimicrobial prescriptions, and medical expenses among children in the United States during 2011-2016. Vaccine 2018; 36:7479–86. [DOI] [PubMed] [Google Scholar]
- 24. Wiese AD, Huang X, Yu C, et al. . Changes in otitis media episodes and pressure equalization tube insertions among young children following introduction of the 13-valent pneumococcal conjugate vaccine: a birth-cohort based study. Clin Infect Dis 2019; 69:2162–9. [DOI] [PubMed] [Google Scholar]
- 25. Lieberthal AS, Carroll AE, Chonmaitree T, et al. . Clinical practice guideline: the diagnosis and management of acute otitis media. Pediatrics 2013; 131:e964–99. [DOI] [PubMed] [Google Scholar]
- 26. Damoiseaux RA, Rovers MM, Van Balen FA, et al. . Long-term prognosis of acute otitis media in infancy: determinants of recurrent acute otitis media and persistent middle ear effusion. Fam Pract 2006; 23:40–5. [DOI] [PubMed] [Google Scholar]
- 27. Rosenblut A, Napolitano C, Pereira A, et al. . Etiology of acute otitis media and serotype distribution of Streptococcus pneumoniae and Haemophilus influenzae in Chilean children <5 years of age. Medicine 2017; 96:e5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parra MM, Aguilar G, Echaniz-Aviles G, et al. . Bacterial etiology and serotypes of acute otitis media in Mexican children. Vaccine 5549; 29:5544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.