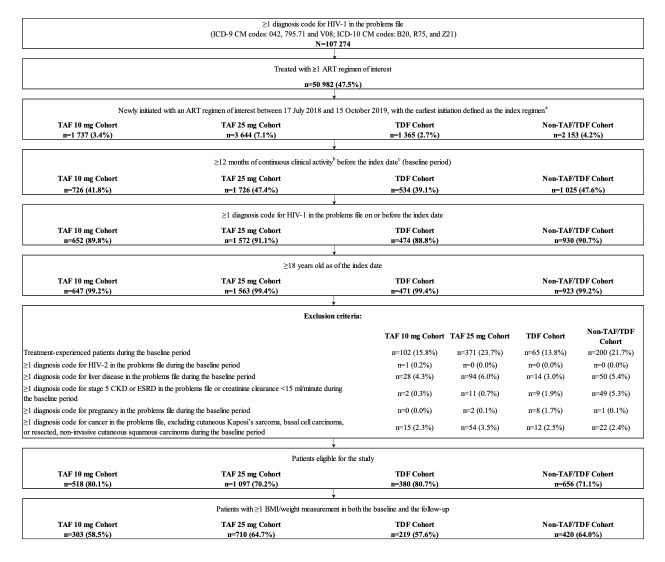
Figure 1. Identification of the Study Population.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CKD, chronic kidney disease; EMR, electronic medical records; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; ICD-9 CM/ICD-10 CM, International Classification of Disease, Ninth/Tenth Revision, Clinical Modification; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
a The index period spanned from July 17, 2018 (date of darunavir/cobicistat/emtricitabine/TAF approval), to October 15, 2019, to allow sufficient follow-up time to observe weight or BMI measurements during the observation period.
b Continuous clinical activity was defined as the period from the first to last record in the EMR database.
c For multi-tablet regimens (MTRs) identified as part of the TAF 10 mg, TAF 25 mg, or TDF cohorts, the index date was the date of the prescription for the TAF 10 mg, TAF 25 mg, or TDF agent used as part of the MTR. For MTRs identified as part of the non-TAF/TDF cohort, the index date was the date that regimen identification was complete.