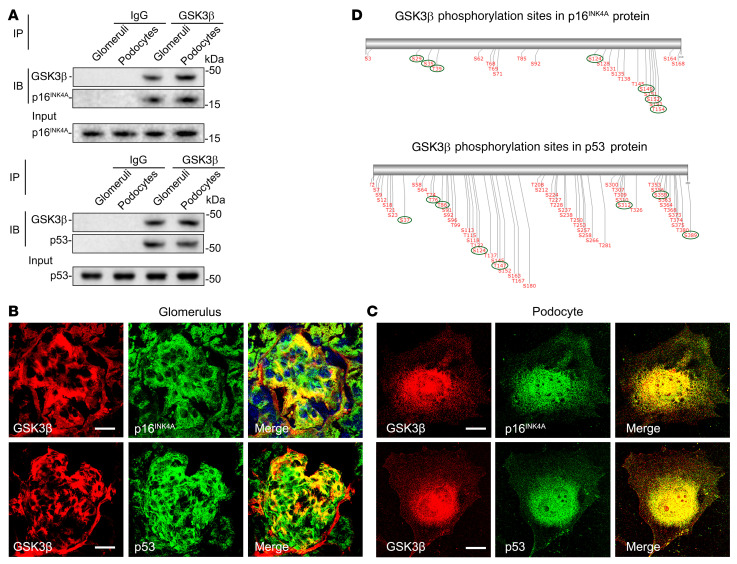
Figure 5. p16INK4A and p53 colocalize and physically interact with GSK3β in glomerular podocytes as its putative substrates.
(A) Lysates of differentiated immortalized murine podocytes and homogenates of glomeruli isolated from WT mice were processed for immunoprecipitation (IP) by using an anti-GSK3β antibody or preimmune IgG, followed by immunoblot analysis (IB) of immunoprecipitates for GSK3β, p16INK4A, and p53 in parallel with input controls. (B and C) Dual-color fluorescent immunostaining for GSK3β (red) and p16INK4A (green) or p53 (green) in (B) mouse kidney tissues as revealed by fluorescence microscopy or in (C) cultured murine podocytes as shown by laser scanning confocal fluorescence microscopy. Scale bars: 20 μm. (D) In silico analysis reveals serine/threonine residues in putative consensus motifs for phosphorylation by GSK3β in p16INK4A and p53. The serines and threonines with high prediction scores for GSK3β consensus motifs are marked with green circles.