Figure 1.
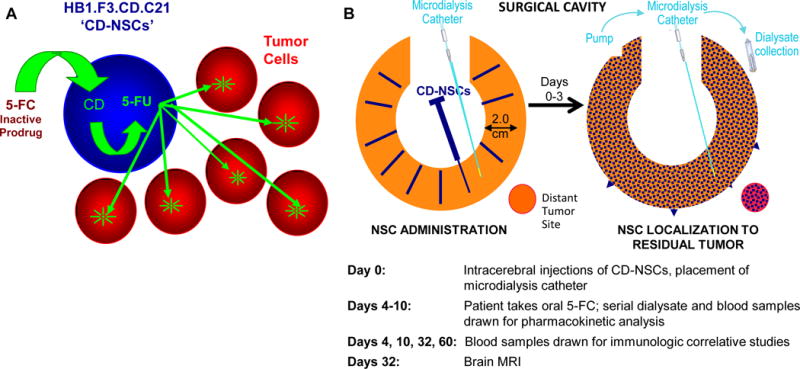
Treatment strategy for CD-NSCs in combination with 5-FC. A. Schematic of tumor-localized production of chemotherapy by CD-NSCs. Intracranially administered CD-NSCs migrate to residual tumor. The CD-NSCs stop dividing within 48 h of administration, allowing them to survive exposure to cytotoxic chemotherapy. The orally administered prodrug, 5-FC, crosses the blood-brain barrier, and the CD expressed in the NSCs converts the 5-FC into 5-FU. The 5-FU then diffuses from the NSCs and kills nearby dividing tumor cells. B. Study treatment schema. On the day of surgery (day 0), the total dose of CD-NSCs was distributed in 100–150 μl volumes and injected throughout the wall of the resection cavity (or as a single 1 ml intratumoral injection via the biopsy track [not shown]). An intracerebral microdialysis catheter was also placed in tumor or peritumoral tissue. Four days later, the patient started taking 5-FC orally every 6 h for 7 days. During this time, dialysate and blood samples were drawn to measure levels of 5-FC and 5-FU. Serial blood samples were also drawn through day 60 to assess for possible anti-NSC antibody and T cell responses and investigate if NSCs migrated into the systemic circulation. A brain MRI was performed at the end of the toxicity evaluation period, 32 days after injection of CD-NSCs.
