Figure 3. Assessment of antibody responses to multiple doses of NSCs.
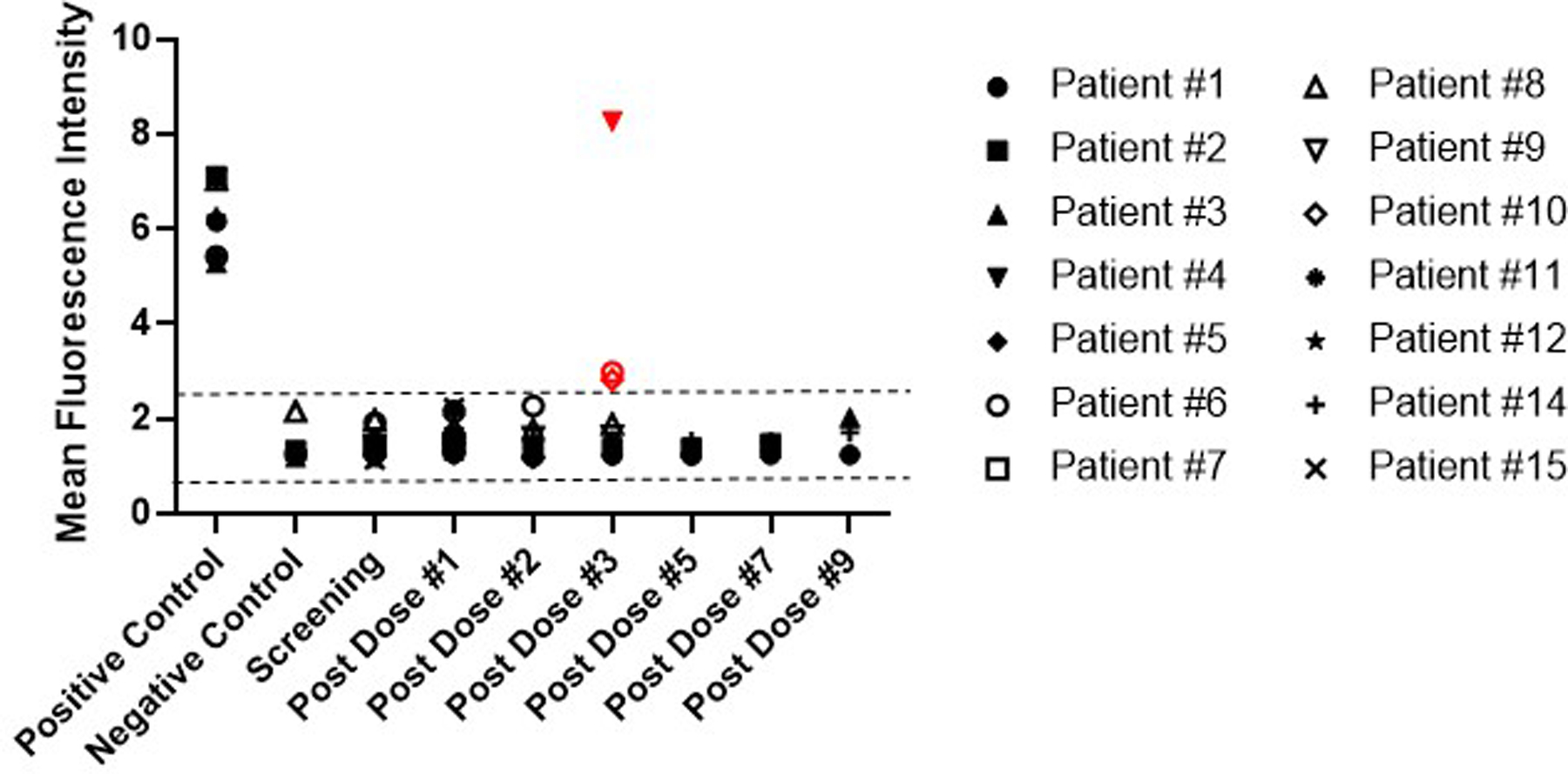
Patient serum samples were assessed by flow cytometry for the presence of anti-NSC antibodies at the time of enrollment, every 2 weeks (prior to administering the first four doses of CD-NSCs) for the first 2 months, and then monthly until the study treatment was stopped. The positive and negative controls (N=5 each) represent the results of 5 independent experiments and consisted of serum from subjects determined to have antibodies against the HLA-I antigens expressed on the CD-NSCs (positive control) and normal donors who did not express HLA-I antigens on the NSCs (negative control). All data points represent the mean of triplicate assays. The dashed lines indicate the range for a “negative” mean fluorescence intensity (MFI) determined from 10 normal donors, resulting in a range of 0.55 to 2.59 and representing a 95% confidence interval based on 2.3 SE around the population mean. The three positive antibody responses are indicated in red (Patients 4, 6, and 10). Patient 13 received only one dose of CD-NSCs and was not included in this analysis. Patient 16 was not included in this analysis either because we were unable to obtain a post second CD-NSC dose serum sample to analyze.
