Abstract
Aims
Isolated tricuspid valve surgery (ITVS) is considered to be a high-risk procedure, but in-hospital mortality is markedly variable. This study sought to develop a dedicated risk score model to predict the outcome of patients after ITVS for severe tricuspid regurgitation (TR).
Methods and results
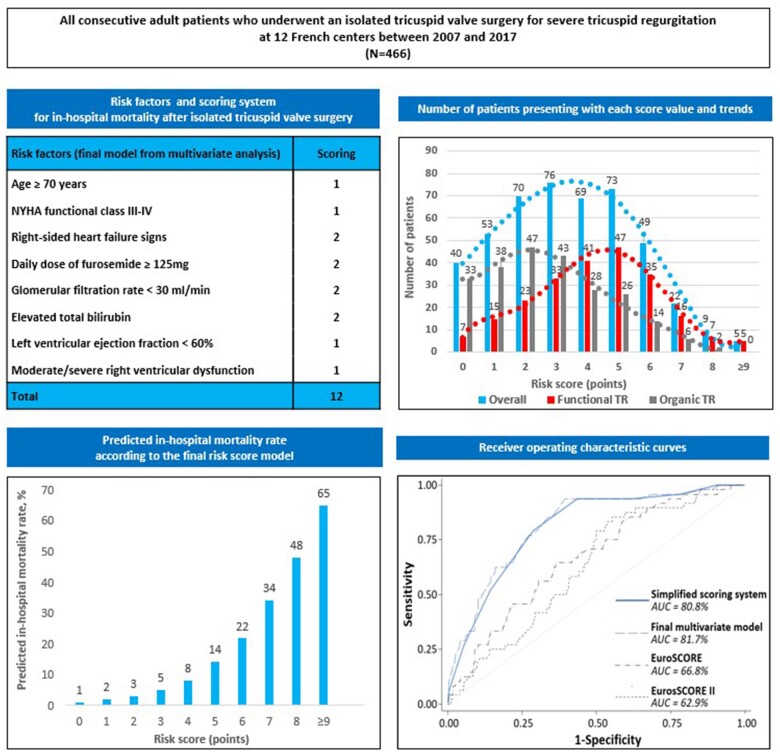
All consecutive adult patients who underwent ITVS for severe non-congenital TR at 12 French centres between 2007 and 2017 were included. We identified 466 patients (60 ± 16 years, 49% female, functional TR in 49%). In-hospital mortality rate was 10%. We derived and internally validated a scoring system to predict in-hospital mortality using multivariable logistic regression and bootstrapping with 1000 re-samples. The final risk score ranged from 0 to 12 points and included eight parameters: age ≥70 years, New York Heart Association Class III–IV, right-sided heart failure signs, daily dose of furosemide ≥125 mg, glomerular filtration rate <30 mL/min, elevated bilirubin, left ventricular ejection fraction <60%, and moderate/severe right ventricular dysfunction. Tricuspid regurgitation mechanism was not an independent predictor of outcome. Observed and predicted in-hospital mortality rates increased from 0% to 60% and from 1% to 65%, respectively, as the score increased from 0 up to ≥9 points. Apparent and bias-corrected areas under the receiver operating characteristic curves were 0.81 and 0.75, respectively, much higher than the logistic EuroSCORE (0.67) or EuroSCORE II (0.63).
Conclusion
We propose TRI-SCORE as a dedicated risk score model based on eight easy to ascertain parameters to inform patients and physicians regarding the risk of ITVS and guide the clinical decision-making process of patients with severe TR, especially as transcatheter therapies are emerging (www.tri-score.com).
Keywords: Risk score, Tricuspid regurgitation, Surgery, Outcome
Graphical Abstract
Introduction
In the community, prevalence of significant—moderate or severe—tricuspid regurgitation (TR) is estimated to be as high as 0.55% and up to 3% after 75 years, a prevalence that is similar to that of aortic stenosis or mitral regurgitation.1 Epidemiological studies suggest that moderate or severe TR affects over 1.6 million individuals in the USA1 and is associated with a twofold increased cardiac mortality that persists after adjustment for potential confounders.2 , 3
Despite its high prevalence and its association with a dismal prognosis, TR remains often conservatively managed and the number of tricuspid valve (TV) surgeries, mostly performed at the time of mitral valve surgery, is remarkably low.4–7 If the management of significant TR at the time of mitral surgery is relatively well codified, the management of patients with isolated—organic or functional—TR is less established.8 , 9 Isolated tricuspid valve surgery (ITVS) is rarely performed (few hundred interventions yearly in the USA)4–6 based upon its reported high mortality rates. If the literature is consistent showing that overall in-hospital mortality is ∼10% both in the USA and in Europe, we have previously shown that it was markedly variable and that it was predicted by the severity of the clinical presentation while TR mechanism/aetiology had a limited impact.4
With the recent development of transcatheter interventions, there is a critical need to accurately predict in-hospital mortality rates for ITVS that could be used in routine practice and support clinical decisions. Both the Society of Thoracic Surgeons (STS) cardiac surgery risk model and the logistic EuroSCORE/EuroSCORE II were not designed to predict outcomes of this type of rare interventions. There have been few attempts to develop a specific risk score model for ITVS,10 but so far an accurate and dedicated risk score model capturing main parameters of interest in the setting of TR is cruelly lacking. In particular, no score has integrated liver function or right ventricular (RV) consequences, which are main prognostic factors in TR, in a risk score model for ITVS.
Relying on a multicentre consecutive cohort of patients who underwent ITVS for severe TR at 12 French tertiary centres, we aimed to develop a dedicated risk score model to accurately predict the outcome of patients after ITVS.
Methods
Study design
Using the local Programme de Médicalisation des Systèmes d’Information (PMSI) database,11 among 5661 consecutive cases of TV surgery performed in adult patients at 12 tertiary French centres between 1 January 2007 and 31 December 2017, we identified 466 patients who underwent an ITVS on native valve for severe non-congenital TR. The design of the study has been previously reported.4 Clinical information and immediate and mid-term outcome were retrospectively collected locally at each centre. Right-sided heart failure signs were defined as severe jugular venous distention, ascites, and/or marked peripheral oedema. Tricuspid regurgitation mechanism was classified as functional (no intrinsic TV disease) or organic (due to abnormalities of the tricuspid leaflets or chordae). Glomerular filtration rate was calculated using the Cockcroft–Gault formula according to sex. Left ventricular (LV) ejection fraction was measured visually or using the biplane method of discs (modified Simpson’s rule). Right ventricular size was visually assessed as normal, mildly, moderately, or severely enlarged. Right ventricular function was semi-quantitatively assessed using an integrative multi-parametric approach, as recommended.12–14 Moderate/severe RV dysfunction was defined by a tricuspid annular plane systolic excursion (TAPSE) <17 mm and/or a Doppler tissue imaging peak systolic annular velocity S′ <9.5 cm/s and/or a markedly reduced fractional area change based on visual assessment. Logistic EuroSCORE and EuroSCORE II were calculated.15 , 16 The Charlson index was used to assess patient comorbidities. Urgent surgery was defined as non-elective and within the same hospital admission. Centres were divided into quartiles according to average yearly number of interventions performed. The study was approved by each local institutional review board.
Outcome
In-hospital mortality was defined as death occurring between the intervention and hospital discharge during the same hospital stay. Major post-operative complications were defined as death, shock, tamponade, acute renal failure requiring dialysis, or prolonged (>72 h) mechanical ventilation. One-year mortality was also collected based on hospital chart reviews and telephone interviews with patients or family members.
Statistical analysis and risk score modelling
For risk score development, variables associated at the P-value <0.2 level in univariate logistic regression were entered into multivariate analysis, using a stepwise backward procedure based on the Akaike information criterion (AIC) for variable selection, sequentially removing items until the best final model with the lowest AIC was obtained. For the sake of simplicity of use, continuous variables were dichotomized before incorporation into regression analyses by means of recursive partitioning analysis and/or accounting for clinically relevant thresholds.17 Regression coefficients of the final model were then considered for use as weights to compute a simplified scoring system, by multiplying and rounding coefficients to their closest integer, following the approach from Cole to determine the optimal multiplier.18 Model discrimination was evaluated by the area under the receiver operating characteristic curve (AUROC), and calibration by plotting calibration curves to evaluate the concordance between observed and predicted survival probability. Internal validation was performed using 1000 bootstrap re-samples, providing bias-corrected AUROC and calibration curves to estimate and account for the amount of optimism in model discrimination and calibration.19 To reduce potential bias arising from complete-case analysis, all regression models were performed after missing data imputation using the non-parametric missForest method20 and Rubin’s rules.21 Additional details are given in Supplementary material online, Methods, regarding the missing data imputation and modelling strategy.
Analyses were performed at the two-tailed P-value <0.05 level, using Stata v16.1 (StataCorp, College Station, TX, USA) for descriptive analyses, and R v4.0.3 (R Foundation for Statistical Computing, Vienna, Austria; packages party, MASS, rms, missForest) for missing data imputation and model development and validation.
Results
Study population
The characteristics of the 466 consecutive patients who underwent ITVS on native valve at our 12 tertiary French centres during the 10-year period are presented in Table 1 and Supplementary material online, Table S1. Briefly, the mean age was 60 ± 16 years, 229 patients (49%) were female, and TR mechanism was functional in 229 patients (49%) and organic in 237 patients (51%). Overall, in-hospital mortality rate was 10% (n = 48), major post-operative complications rate was 31% (n = 145), and 1-year mortality rate was 12% (n = 55). In-hospital and 1-year follow-up were 100% and 90% complete, respectively.
Table 1.
Baseline characteristics overall and according to discharge vital status (raw data only), and univariate logistic regression (with imputed data) for predictors of in-hospital mortality
| Characteristics | Overall (n = 466) | By vital status |
Univariate |
||||
|---|---|---|---|---|---|---|---|
| Discharged alive (n = 418) | In-hospital death (n = 48) | P-value | Odds ratio | 95% CI | P-value | ||
| Age (years) | 60 ± 16 | 59 ± 16 | 68 ± 12 | <0.001 | 1.05 | 1.02–1.07 | <0.001 |
| Age ≥70 years | 141 (30) | 117 (28) | 24 (50) | 0.003 | 2.57 | 1.41–4.71 | 0.003 |
| Female sex | 229 (49) | 205 (49) | 24 (50) | 1.00 | 1.04 | 0.57–1.89 | 0.90 |
| Body mass index (kg/m2) | 25 ± 5 | 25 ± 5 | 26 ± 6 | 0.66 | 1.02 | 0.96–1.07 | 0.60 |
| Hypertension | 190 (41) | 166 (40) | 24 (50) | 0.22 | 1.52 | 0.83–2.76 | 0.18 |
| Diabetes mellitus | 62 (13) | 56 (13) | 6 (13) | 1.00 | 0.92 | 0.38–2.27 | 0.90 |
| Chronic lung disease | 51 (11) | 43 (10) | 8 (17) | 0.27 | 1.74 | 0.77–3.97 | 0.20 |
| Peripheral vascular disease | 16 (3) | 14 (3) | 2 (4) | 0.68 | 1.25 | 0.28–5.69 | 0.73 |
| Prior stroke | 41 (9) | 38 (9) | 3 (6) | 0.79 | 0.67 | 0.20–2.25 | 0.55 |
| Prior left-sided heart valve surgery | 111 (24) | 95 (23) | 16 (33) | 0.15 | 1.70 | 0.89–3.23 | 0.11 |
| Coronary artery disease | 59 (13) | 50 (12) | 9 (19) | 0.27 | 1.70 | 0.78–3.72 | 0.20 |
| Chronic kidney disease | 154 (33) | 134 (32) | 20 (42) | 0.24 | 1.51 | 0.82–2.78 | 0.19 |
| Permanent pacemaker | 104 (22) | 88 (21) | 16 (33) | 0.08 | 1.88 | 0.98–3.57 | 0.06 |
| Hospitalization for congestive heart failure (<1 year) | 163 (35) | 138 (33) | 25 (52) | 0.01 | 2.21 | 1.21–4.03 | 0.01 |
| Systolic blood pressure (mmHg) | 125 ± 19 | 125 ± 20 | 122 ± 17 | 0.44 | 0.99 | 0.98–1.01 | 0.42 |
| Diastolic blood pressure (mmHg) | 73 ± 13 | 73 ± 13 | 70 ± 14 | 0.21 | 0.98 | 0.95–1.00 | 0.11 |
| NYHA functional Class III–IV | 217 (47) | 184 (44) | 33 (69) | 0.002 | 2.80 | 1.48–5.31 | 0.001 |
| Right-sided heart failure signs | 264 (57) | 223 (53) | 41 (85) | <0.001 | 5.12 | 2.25–11.7 | <0.001 |
| Ascites | 39 (8) | 29 (7) | 10 (21) | 0.003 | 3.53 | 1.60–7.79 | 0.004 |
| Loop diuretics | 301/451 (67) | 260/404 (64) | 41/47 (87) | 0.003 | 3.68 | 1.53–8.86 | 0.001 |
| Daily dose of furosemide (mg) | 40 (0–80) | 40 (0–80) | 80 (40–250) | 0.008 | 1.00 | 1.00–1.00 | <0.0 01 |
| Daily dose of furosemide ≥125 mg | 67 (15) | 48 (12) | 19 (40) | <0.001 | 5.05 | 2.63–9.69 | <0.001 |
| Atrial fibrillation | 181 (39) | 154 (37) | 27 (56) | 0.01 | 2.20 | 1.20–4.03 | 0.01 |
| Haemoglobin (g/dL) | 12.3 ± 2.3 | 12.3 ± 2.3 | 11.9 ± 1.8 | 0.13 | 0.91 | 0.80–1.05 | 0.19 |
| Glomerular filtration rate (mL/min) | 72 ± 39 | 73 ± 40 | 58 ± 30 | 0.003 | 0.99 | 0.98–1.00 | 0.008 |
| Glomerular filtration rate <30 mL/min | 32/442 (7) | 24/398 (6) | 8/44 (18) | 0.003 | 3.28 | 1.38–7.79 | <0.001 |
| Elevated ALT and/or AST | 73/393 (19) | 62/355 (18) | 11/38 (29) | 0.13 | 1.88 | 0.95–3.73 | 0.08 |
| Elevated GGT and/or ALP | 189/397 (48) | 164/359 (46) | 25/38 (66) | 0.03 | 2.59 | 1.38–4.87 | 0.003 |
| Elevated total bilirubin | 143/389 (37) | 119/352 (34) | 24/37 (65) | <0.001 | 3.78 | 2.03–7.02 | <0.001 |
| Left ventricular ejection fraction (%) | 58 ± 9 | 58 ± 9 | 54 ± 9 | 0.009 | 0.96 | 0.93–0.99 | 0.005 |
| Left ventricular ejection fraction <60% | 199/452 (44) | 169/404 (42) | 30 (63) | 0.01 | 0.38 | 0.19–0.79 | 0.006 |
| Moderate/severe right ventricular dilatation | 245/450 (54) | 212/402 (53) | 33 (69) | 0.05 | 2.08 | 1.10–3.94 | 0.02 |
| TAPSE (mm) | 20 ± 7 | 20 ± 7 | 17 ± 6 | 0.004 | 0.90 | 0.85–0.95 | <0.001 |
| Peak systolic annular velocity S′ (cm/s) | 11.9 ± 4.1 | 12.1 ± 4.1 | 10.7 ± 3.3 | 0.04 | 0.83 | 0.74–0.92 | 0.001 |
| Moderate/severe right ventricular dysfunction | 76/446 (17) | 60/398 (15) | 16 (33) | 0.003 | 2.98 | 1.54–5.77 | 0.002 |
| Tricuspid annulus diameter (mm) | 44 ± 9 | 44 ± 8 | 46 ± 8 | 0.30 | 1.04 | 1.00–1.08 | 0.07 |
| Systolic pulmonary artery pressure (mmHg) | 40 ± 11 | 40 ± 11 | 45 ± 10 | 0.01 | 1.05 | 1.02–1.09 | 0.001 |
| Systolic pulmonary artery pressure ≥50 mmHg | 52/274 (19) | 42/247 (17) | 10/27 (37) | 0.02 | 1.93 | 0.80–4.63 | 0.16 |
| Functional aetiology of the tricuspid regurgitation | 229 (49) | 196 (47) | 33 (69) | 0.007 | 2.49 | 1.31–4.73 | 0.004 |
| Logistic EuroSCORE | 5.2 (3.0–9.2) | 5 (2.8–8.6) | 7.6 (4.4–15.9) | 0.01 | 1.05 | 1.02–1.08 | 0.002 |
| EuroSCORE II | 2.7 (1.4–5.0) | 2.5 (1.3–5) | 3.7 (2.5–6.7) | 0.09 | 1.03 | 0.98–1.08 | 0.21 |
| Charlson comorbidity index | 3 (1–4) | 3 (1–4) | 4 (3–6) | <0.001 | 1.29 | 1.14–1.45 | <0.001 |
| Charlson comorbidity index ≥2 | 324 (70) | 281 (67) | 43 (90) | 0.003 | 4.19 | 1.62–10.8 | 0.001 |
| Urgent surgery | 102 (22) | 93 (22) | 9 (19) | 0.71 | 0.81 | 0.38–1.73 | 0.60 |
| Beating heart | 98 (25) | 84 (23) | 14 (37) | 0.10 | 1.57 | 0.81–3.05 | 0.20 |
| Tricuspid valve replacement | 273 (59) | 240 (57) | 33 (69) | 0.18 | 1.63 | 0.86–3.10 | 0.13 |
| Major post-operative complications | 145 (31) | — | — | — | — | — | — |
| Mortality at 1 year | 55 (12) | — | — | — | — | — | — |
Values are mean ± standard deviation, n (%), or median (interquartile range); bolded results are statistically significant at the P-value <0.05 level.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; GGT, gamma-glutamyl transferase; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion.
Univariate analysis
Factors associated with in-hospital death in univariate analysis are presented in Table 1, showing comparisons according to final vital status based on raw data in the left section, and unadjusted odds ratios from logistic regression on imputed data in the right section. In-hospital mortality was statistically significantly associated with older age and more severe presentation based on clinical, laboratory, and echocardiographic assessment such as history of congestive heart failure, severe symptoms [New York Heart Association (NYHA) Class III/IV, right-sided heart failure signs), dose of furosemide, kidney or liver impairment, TR mechanism, degree of RV dilatation and dysfunction, but not with previous left-sided heart valve surgery (P = 0.11). When centres were divided into quartiles according to average yearly number of interventions, we did not observe an impact of centre volume on in-hospital mortality (from the lowest-volume quartile to the highest-volume quartile: 13%, 9%, 12%, and 9%, respectively; P = 0.83).
Predictive model development and scoring
Variables associated at the P-value < 0.2 level in univariate analysis were entered into multivariate modelling. To develop a simplified scoring system, continuous variables were dichotomized before incorporation into regression analyses and were defined as follows: age ≥70 years, daily dose of furosemide ≥125 mg, glomerular filtration rate <30 mL/min, abnormal total bilirubin, alanine aminotransferase/aspartate aminotransferase and gamma-glutamyl transferase/alkaline phosphatases levels, LV ejection fraction <60%, systolic pulmonary artery pressure ≥50 mmHg and Charlson comorbidity index ≥2. Using stepwise multivariate analysis based on AIC, eight variables were selected in the final model: age, NYHA functional Class III–IV, right-sided heart failure signs, daily dose of furosemide, glomerular filtration rate, total bilirubin level, LV ejection fraction and moderate/severe RV dysfunction. In the final simplified scoring model, 1 point was attributed to a risk factor when odds ratio was between 1 and 2 and 2 points when odds ratio was ≥2 (Table 2).
Table 2.
Risk factors for in-hospital mortality: final model from multivariate analysis and scoring system
| Risk factors | Odds ratio | 95% CI | Regression coefficient | Final scoring |
|---|---|---|---|---|
| Age ≥70 years | 1.65 | 0.84–3.21 | 0.50 | 1 |
| NYHA functional Class III–IV | 1.76 | 0.88–3.55 | 0.57 | 1 |
| Right-sided heart failure signs | 2.62 | 1.08–6.35 | 0.96 | 2 |
| Daily dose of furosemide ≥125 mg | 2.25 | 1.08–4.68 | 0.81 | 2 |
| Glomerular filtration rate <30 mL/min | 2.47 | 0.92–6.62 | 0.90 | 2 |
| Elevated total bilirubin | 2.89 | 1.48–5.63 | 1.06 | 2 |
| Left ventricular ejection fraction <60% | 1.97 | 0.91–4.28 | 0.68 | 1 |
| Moderate/severe right ventricular dysfunction | 1.93 | 0.93–4.01 | 0.66 | 1 |
| Total | 12 |
CI, confidence interval; NYHA, New York Heart Association.
Predictive model validation and calibration
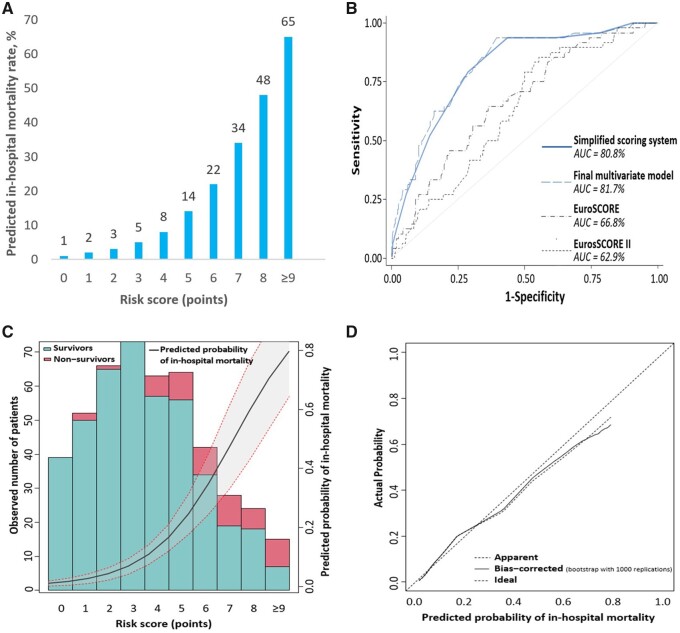
Observed and predicted in-hospital mortality rates according to the score from the risk score model ranged from 0% to 60% and from 1% to 65%, respectively, for a score of 0 to a score of 9 or more, with exponential increased mortality rates as the score increased (Table 3 and Figure 1A). Good discrimination performance was found for the final simplified risk score model with an apparent AUROC of 0.808 (0.817 for the raw regression model without scaling/rounding of coefficients) and a bias-corrected AUROC of 0.753 (0.762) from internal validation. These results were found to be substantially higher than those obtained with both logistic EuroSCORE and EuroSCORE II (AUROC 0.668 and 0.629, respectively) (Figure 1B and C). Calibration of the risk score model was appropriate, as shown in calibration curves demonstrating very good agreement between actual and predicted probability of death for probabilities up to 50%, with a slight overestimation of the model for higher probabilities (Figure 1D).
Table 3.
Predicted vs. observed in-hospital mortality rates according to the risk score value
| Score | Number of patients | Predicted in-hospital mortality (%) | Observed in-hospital mortality (%) |
|---|---|---|---|
| 0 | 40 | 1 | 0 |
| 1 | 53 | 2 | 4 |
| 2 | 70 | 3 | 1 |
| 3 | 76 | 5 | 0 |
| 4 | 69 | 8 | 10 |
| 5 | 73 | 14 | 18 |
| 6 | 49 | 22 | 25 |
| 7 | 22 | 34 | 32 |
| 8 | 9 | 48 | 33 |
| ≥9 | 5 | 65 | 60 |
Figure 1.
Discrimination and calibration of the risk score model. (A) Predicted in-hospital mortality rate according to the final risk score model. (B) Receiver operating characteristic curves from final multivariate risk score model, simplified risk score model, logistic EuroSCORE, and EuroSCORE II. (C) Calibration of the final multivariate risk score model: scores vs. probability of in-hospital mortality. (D) Calibration of the final multivariate risk score model: predicted vs. actual probability of in-hospital mortality. AUC: area under the curve.
Distribution of the risk score model according to tricuspid regurgitation mechanism
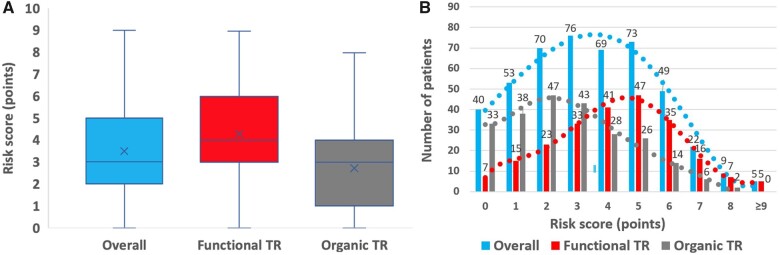
Overall, mean score value was 3.5 ± 2.1 [median: 3 (2–5)] (Figure 2A) and distribution (number of patients presenting with each score value) is presented in Figure 2B. In-hospital mortality was worse for functional than for organic TR, but the mechanism was not an independent predictor of outcome and worse outcome observed in patients with functional TR was related to the worse clinical presentation in this subgroup. Thus, patients with functional TR were operated with a higher-risk score than patients with organic TR {4.3 ± 2.0 [median: 4 (3–6)] vs. 2.7 ± 1.9 [median: 3 (1–4)], P < 0.001} (Figure 2A) and there was a shift towards the right of the distribution of the score in patients with functional TR compared to those with organic TR (Figure 2B).
Figure 2.
Risk score value and distribution overall and according to the mechanism of tricuspid regurgitation. (A) Box plot of risk score value. Within each box, the horizontal line denotes the median value (50th percentile) and the cross the mean; boxes extend from the 25th to the 75th percentile of dataset. The whiskers mark the minimum and maximum values. (B) Number of patients presenting with each score value and trends. TR: tricuspid regurgitation.
Predictive value of the score for in-hospital major complications and 1-year mortality
Although the risk score model was originally designed to predict in-hospital mortality, it was also significantly associated with major post-operative complications (C-index 0.71) and 1-year mortality rates (C-index 0.78). Major post-operative complications and 1-year mortality rates according to the score value ranged from 15% to 80% and from 3% to 60%, respectively, for a score value of 0 to a score of 9 or more, with a progressive increase in event rates as the score increased (Supplementary material online, Table S2).
Discussion
In this study, based on a large consecutive cohort of patients who underwent ITVS for severe TR at 12 French tertiary centres with in-depth clinical, laboratory, and echocardiographic characterization, we developed a dedicated risk score model to predict in-hospital mortality. The risk score model relied on eight parameters, four clinical parameters (age ≥70 years, NYHA functional Class III–IV, right-sided heart failure signs, daily dose of furosemide ≥125 mg), two laboratory parameters (glomerular filtration rate <30 mL/min, elevated total bilirubin), and two echocardiographic parameters (LV ejection fraction <60%, moderate/severe RV dysfunction) (Graphical Abstract). The risk score model showed both an excellent discrimination and calibration. Although designed to predict in-hospital mortality, our risk score model also predicted in-hospital major complication rates and 1-year mortality rates with good accuracy.
Graphical Abstract.
TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. AUC: area under the curve; TR: tricuspid regurgitation.
Despite its high prevalence and association with increased mortality and morbidity, surgery for isolated TR remains rarely performed. Thus, only 10% of patients admitted in France with a diagnosis of TR are referred for an intervention.7 Management of patients with severe TR relied on two apparent contradictory beliefs, on the one hand that TR is benign and on the other hand that surgical risk for ITVS is prohibitive. In contrast to aortic stenosis or mitral regurgitation, TR is a slowly progressive disease and adverse consequences often occurred after decades explaining its falsely benign reputation.22 It is also true that the literature is fairly consistent showing an overall high in-mortality rate of 8–10%.4–6 , 10 , 23–25 We also observed that overall in-hospital mortality rate was indeed 10%,4 but in the present paper we clearly showed that this 10% mortality rate is hiding important disparities with in-hospital mortality rates ranging from 0% to 60%. We have previously shown that outcomes were predicted by the severity of the presentation while TR mechanism or aetiology played a limited role. However, our findings were of limited use to predict immediate and mid-term results of ITVS at the individual level and a risk score model that could support clinical decisions for cardiologists and cardiac surgeons was cruelly lacking.
The STS risk score model does not individualize ITVS as a specific intervention contrary to aortic or mitral valve surgeries and thus does not provide any specific risk calculation. The logistic EuroSCORE and the EuroSCORE II were not designed for ITVS; interestingly the EuroSCORE II population only encompassed 85 patients who underwent ITVS. Consequently, as shown in the present study, both the logistic EuroSCORE and the EuroSCORE II poorly predicted in-hospital mortality after ITVS (C-index 0.67 and 0.63, respectively). In an attempt to better assess the surgical risk of ITVS, LaPar et al. 10 proposed a dedicated risk score model derived from a subset of the STS database from the states of Virginia and Michigan. Although the authors should be commended for their seminal work and indeed their risk model was an important step towards a better risk stratification of patients referred for ITVS, it suffered from two important limitations. First, because of the limited granularity of the STS database, important prognostic factors in the setting of TR as RV consequences and liver function were not accounted for.26 Second, their risk model was not well calibrated for high-risk patients with a maximum predicted mortality rate of 34%. The Model for End-Stage Liver Disease (MELD) score, which is used to stratify patients awaiting liver transplantation, has also been shown to predict mortality after TV surgery in a cohort of 168 patients but in whom only 22 patients underwent ITVS.27 , 28 The MELD relies on three variables that reflect liver and renal function [international normalized ratio (INR), total bilirubin, and creatinine]. However, it is worth noting that this score may be unsuitable for most patients who underwent ITVS as the majority of patients in our population were under oral anticoagulation treatment (vitamin K antagonist or direct oral anticoagulant), which increases the INR, and thus make this score likely not interpretable.
In contrast, in the present study, relying on the in-depth characterization of our population, we were able to develop a dedicated, simple, and accurate risk score model for ITVS that could guide the clinical decision-making process. We collected all consecutive patients who underwent ITVS for severe TR at 12 tertiary French centres. Our risk score model was based on eight clinical, laboratory and echocardiographic parameters easy to capture and to measure, including those capturing the clinical sequalae of TR on the liver and the right ventricle. Our risk score model, on a 0–12 point scale, provided both excellent discrimination (C-index > 0.75) and calibration with a predicted mortality up to 65%. Interestingly, systolic pulmonary artery pressure was not an independent predictor of outcome. In the setting of severe TR, a laminar flow is commonly observed, and echocardiographic assessment of systolic pulmonary artery pressure may be not valid or may be underestimated in this situation. It is also worth noting that as patients with functional TR presented with more advanced disease than patients with organic TR, their risk score was significantly higher and the distribution of the proportion of patients with each score value was right-shifted (Figure 2). Importantly, our risk score model designed to predict in-hospital mortality also provided good predictive value for both in-hospital major complications and 1-year mortality rates that are also critical to appraise when ITVS is considered.
Study limitations
The present study deserves several comments. First, our sample size was relatively small (466 patients), but ITVS is a rare intervention. Our population, one of the largest to date, captured all ITVS performed at 12 centres during a period of >10 years representing >40% of all ITVS at the national level with in-depth clinical, laboratory and echocardiographic characterization. Nevertheless, we were not able to develop and validate a risk score model in different population subsets and further external validation of our score is desirable. Second, our study was retrospective but the low rate of ITVS precluded the prospective collection of a large number of patients referred for ITVS. Third, not all variables were available for each patient, and we performed imputation for missing variables. However, we performed multiple sensitivity analyses that found similar results; it is also noteworthy that all variables retained in the final model had clinical relevance, thus supporting the robustness of our findings. Fourth, we voluntarily did not use prothrombin time in our score as most patients were under oral anticoagulation treatment in our cohort, which can mislead its interpretation, especially if we consider the increased use of direct oral anticoagulants in recent years. Fifth, assessment of RV function relied on an integrative approach and not a single parameter with a well-defined threshold. However, each echocardiographic parameter proposed for the assessment of RV systolic function suffers from intrinsic limitations and have not been well validated in the setting of severe TR. Thus, an integrative approach was deemed the most accurate and reliable way to assess RV systolic function and is the recommended approach.12 , 13 , 27 As an internal validation, TAPSE and peak systolic annular velocity S′ were markedly different between patients who presented with moderate/severe RV dysfunction compared to those who did not (13 ± 4 vs. 21 ± 6 mm and 7.6 ± 1.5 vs. 12.4 ± 3.2 cm/s respectively, both P < 0.0001). In addition, RV function, as the other echocardiographic parameters, was assessed locally by each centre with no centralized evaluation. Finally, we cannot exclude a centre effect on outcome after ITVS, but the very low rate overall (from two to six ITVS/year/centre) precludes any formal conclusion.
Clinical implications
The main aim of our risk score model is to provide reliable information to patients, cardiologists, and cardiac surgeons regarding the risk of ITVS for severe TR at an individual level and to guide the clinical decision-making process. The present score model is easy to use and calculate as relying on eight parameters that are part of the routine examination of all patients with severe TR. It helps stratifying the mortality risk when an isolated TV intervention is considered. A risk score ≤3 could define a low surgical risk, a score of 4–5 an intermediate risk, and a score ≥6 a high surgical risk. To enable wide use of this new score (TRI-SCORE), we have developed an online calculator to support physicians in their risk assessment (www.tri-score.com).
Severe TR is associated with a dismal prognosis with progressive RV dysfunction, renal and liver failure, chronic right heart failure, and need for increasing doses of diuretics. In the present study, we captured the full spectrum of disease stages, and we clearly show that a substantial proportion are still referred for an intervention late in the course of the disease with high scores (30% at intermediate risk and 18% at high risk based on the above proposed thresholds) and therefore high mortality rates. On the other hand, excellent outcomes can be achieved when the intervention is timely performed early in the course of the disease with a low score. Thus, the paradigm should be shifted, the poor outcome of ITVS observed overall is not related to the complexity of the procedure per se but to the late referral and the advanced disease stage of many patients. Our results should be seen as a strong incentive to consider a TV intervention earlier in the disease course avoiding performance of TV intervention in patients at a late or desperate disease stage. Nevertheless, we only provided indirect evidence that earlier intervention is likely beneficial and best timing remained to be determined.
With the rapid development of transcatheter interventions, our risk score model will provide a unique tool to select the patients who might be better suitable for surgery or for transcatheter interventions. Importantly, most patients with severe TR still remain conservatively managed. Transcatheter therapies, as a less-invasive alternative to surgery, will further push for an early intervention as well as for an extension of the number of patients treated if proved to be safe and efficient. It is also worth noting that availability of transcatheter TV replacement might circumvent one main limitation of edge-to-edge repair, i.e. significant residual TR. Ongoing and future randomized controlled trials (TRILUMINATE Pivotal Trial, TRI-FR, CLASP II TR, TRISCEND II Pivotal trial) will hopefully provide more evidence regarding most appropriate timing and recommended type of intervention to improve the outcome of this population.
Finally, the main accepted explanation of the discordant results between MITRA-FR and COAPT trials is the enrolment of different subsets of patients with functional mitral regurgitation.29 , 30 TRI-SCORE will provide a unique tool to characterize and compare populations enrolled in ongoing randomized controlled trials evaluating the benefit of TR correction.
Conclusion
We propose TRI-SCORE as a dedicated risk score model based on eight easy to ascertain parameters to inform both patients and physicians regarding the risk of ITVS. This risk score will guide the clinical decision-making process at the bedside level, and we do hope leads to earlier and wider interventions for patients with severe TR before the occurrence of irreversible consequences that markedly affect prognosis, especially as transcatheter therapies are emerging (www.tri-score.com).
Supplementary Material
Acknowledgements
We are grateful to Doctor Samer Nashef and Christopher Smith for giving us access to unpublished data regarding the EuroSCORE.
Conflict of interest: T.M. received consultant fees from Abbott, Edwards, and Medtronic, outside the submitted work. B.I. received consultant fees from Edwards, outside the submitted work. J.-F.O. received consultant fees from Abbott, Carmat, Delacroix-Chevalier, Landanger, Medtronic, and Sorin, outside the submitted work. D.M.-Z. received consultant fees and research grants from Edwards, outside the submitted work. All other authors declared no conflict of interest.
Data availability
Data sharing with qualified researchers may be considered after submission of a proposal to Doctor Julien Dreyfus.
Contributor Information
Julien Dreyfus, Cardiology Department, Centre Cardiologique du Nord, 32-36 rue des moulins gémeaux, Saint-Denis 93200, France.
Etienne Audureau, Public Health Department, AP-HP (Assistance Publique-Hôpitaux de Paris), Henri Mondor University Hospital, 51 Avenue du Maréchal de Lattre de Tassigny, Créteil 94010, France; Univ Paris Est Creteil, INSERM, IMRB, CEpiA Team, Creteil 94010, France.
Yohann Bohbot, Department of Cardiology, Amiens University Hospital, 1 Rue du Professeur Christian Cabrol, Amiens 80054, France; UR UPJV 7517, Jules Verne University of Picardie, 51 Boulevard de Châteaudun, Amiens 80000, France.
Augustin Coisne, CHU Lille, Department of Clinical Physiology and Echocardiography, Heart Valve Clinic, 2 Avenue Oscar Lambret, Lille 59000, France; Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011 - EGID, Lille 59000, France.
Yoan Lavie-Badie, Department of Cardiology, Rangueil University Hospital, 9 Place Lange, Toulouse 31000, France.
Maxime Bouchery, AP-HP (Assistance Publique-Hôpitaux de Paris), Clinical Research Unit (URC Mondor), Henri Mondor University Hospital, 51 Avenue du Maréchal de Lattre de Tassigny, Créteil 94010, France.
Michele Flagiello, Department of Cardiovascular Surgery and Transplantation, Louis Pradel Cardiovascular Hospital, Claude Bernard University, 59 Boulevard Pinel, Bron 69500, France.
Baptiste Bazire, Department of Cardiology, Bichat Claude Bernard Hospital, 46 Rue Henri Huchard, Paris 75018, France.
Florian Eggenspieler, Department of Cardiology, University Hospital of Nancy-Brabois, 29 Avenue du Maréchal de Lattre de Tassigny, Nancy 54000, France.
Florence Viau, Cardiology Department, APHM, La Timone Hospital, 278 Rue Saint-Pierre, Marseille 13005, France.
Elisabeth Riant, Cardiology Department, Centre Cardiologique du Nord, 32-36 rue des moulins gémeaux, Saint-Denis 93200, France; Cardiology Department, Expert Valve Center, Henri Mondor Hospital, 51 Avenue du Maréchal de Lattre de Tassigny, Créteil 94010, France.
Yannick Mbaki, Cardiology Department, CHU de RENNES, LTSI UMR1099, INSERM, Université de Rennes-1, 2 Rue Henri le Guilloux, Rennes 35000, France.
Damien Eyharts, Department of Cardiology, Rangueil University Hospital, 9 Place Lange, Toulouse 31000, France.
Thomas Senage, Department of Cardiac Surgery, INSERM 1246, Université de Nantes, CHU de Nantes, 8 Quai Moncousu, Nantes 44007, France.
Thomas Modine, CHU Lille, Department of Clinical Physiology and Echocardiography, Heart Valve Clinic, 2 Avenue Oscar Lambret, Lille 59000, France.
Martin Nicol, Cardiology Department, Centre Cardiologique du Nord, 32-36 rue des moulins gémeaux, Saint-Denis 93200, France.
Fabien Doguet, Service de chirurgie cardiovasculaire et thoracique, CHU Charles Nicolle, 37 Boulevard Gambetta, Rouen 76000, France; Normandie Univ, Unirouen, INSERM U1096, Rouen 76000, France.
Virginia Nguyen, Cardiology Department, Centre Cardiologique du Nord, 32-36 rue des moulins gémeaux, Saint-Denis 93200, France.
Thierry Le Tourneau, Université de Nantes, CHU de Nantes, CNRS, INSERM, L’institut du thorax, Nantes 44000, France.
Christophe Tribouilloy, Department of Cardiology, Amiens University Hospital, 1 Rue du Professeur Christian Cabrol, Amiens 80054, France; UR UPJV 7517, Jules Verne University of Picardie, 51 Boulevard de Châteaudun, Amiens 80000, France.
Erwan Donal, Cardiology Department, CHU de RENNES, LTSI UMR1099, INSERM, Université de Rennes-1, 2 Rue Henri le Guilloux, Rennes 35000, France.
Jacques Tomasi, Department of Cardiac Surgery, CHU de RENNES, Université de Rennes-1, 2 Rue Henri le Guilloux, Rennes 35000, France.
Gilbert Habib, Cardiology Department, APHM, La Timone Hospital, 278 Rue Saint-Pierre, Marseille 13005, France; Aix Marseille Univ, IRD, APHM, MEPHI, IHU-Méditerranée Infection, 19-21 Boulevard Jean Moulin, Marseille 13005, France.
Christine Selton-Suty, Department of Cardiology, University Hospital of Nancy-Brabois, 29 Avenue du Maréchal de Lattre de Tassigny, Nancy 54000, France.
Richard Raffoul, Department of Cardiac Surgery, AP-HP, Bichat Hospital, 46 Rue Henri Huchard, Paris 75018, France.
Bernard Iung, Cardiology Department, AP-HP, Bichat Hospital, Université de Paris, 46 Rue Henri Huchard, Paris 75018, France.
Jean-François Obadia, Department of Cardiovascular Surgery and Transplantation, Louis Pradel Cardiovascular Hospital, Claude Bernard University, 59 Boulevard Pinel, Bron 69500, France.
David Messika-Zeitoun, Department of Cardiology, University of Ottawa Heart Institute, 40 ruskin street, Ottawa, Ontario, Canada.
Supplementary material
Supplementary material is available at European Heart Journal online.
References
- 1. Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez-Sarano M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019;12:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, Lal S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J 2019;40:476–484. [DOI] [PubMed] [Google Scholar]
- 3. Messika-Zeitoun D, Verta P, Gregson J, Pocock SJ, Boero I, Feldman TE, Abraham WT, Lindenfeld J, Bax J, Leon M, Enriquez-Sarano M. Impact of tricuspid regurgitation on survival in patients with heart failure: a large electronic health record patient-level database analysis. Eur J Heart Fail 2020;22:1803–1813. [DOI] [PubMed] [Google Scholar]
- 4. Dreyfus J, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E, Mbaki Y, Bohbot Y, Eyharts D, Senage T, Dubrulle H, Nicol M, Doguet F, Nguyen V, Coisne A, Le Tourneau T, Lavie-Badie Y, Tribouilloy C, Donal E, Tomasi J, Habib G, Selton-Suty C, Raffoul R, Iung B, Obadia JF, Messika-Zeitoun D. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304–4317. [DOI] [PubMed] [Google Scholar]
- 5. Dreyfus J, Ghalem N, Garbarz E, Cimadevilla C, Nataf P, Vahanian A, Caranhac G, Messika-Zeitoun D. Timing of referral of patients with severe isolated tricuspid valve regurgitation to surgeons (from a French Nationwide Database). Am J Cardiol 2018;122:323–326. [DOI] [PubMed] [Google Scholar]
- 6. Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, Miller VM, Nishimura RA. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–2960. [DOI] [PubMed] [Google Scholar]
- 7. Messika-Zeitoun D, Candolfi P, Dreyfus J, Burwash IG, Iung B, Philippon JF, Toussaint JM, Verta P, Feldman TE, Obadia JF, Vahanian A, Mesana T, Enriquez-Sarano M. Management and outcome of patients admitted with tricuspid regurgitation in France. Can J Cardiol 2021;37:1078–1085. [DOI] [PubMed] [Google Scholar]
- 8. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2021; doi: 10.1093/eurheartj/ehab395. [DOI] [Google Scholar]
- 9. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020. ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-e71. [DOI] [PubMed] [Google Scholar]
- 10. LaPar DJ, Likosky DS, Zhang M, Theurer P, Fonner CE, Kern JA, Bolling SF, Drake DH, Speir AM, Rich JB, Kron IL, Prager RL, Ailawadi G. Development of a risk prediction model and clinical risk score for isolated tricuspid valve surgery. Ann Thorac Surg 2018;106:129–136. [DOI] [PubMed] [Google Scholar]
- 11. PMSI. Programme de Médicalisation des Systèmes d’Information (PMSI). 2012. https://drees.solidarites-sante.gouv.fr/redressements-du-programme-de-medicalisation-des-systemes-d-informations-pmsi.
- 12. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 15. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 16. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–744. [DOI] [PubMed] [Google Scholar]
- 17. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graphical Stat 2006;15:651–674. [Google Scholar]
- 18. Cole T. Scaling and rounding regression coefficients to integers. J R Stat Soc Series C Appl Stat 1993;42:261–268. [Google Scholar]
- 19. Steyerberg E. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer-Verlag; 2009. [Google Scholar]
- 20. Stekhoven DJ, Buhlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 2012;28:112–118. [DOI] [PubMed] [Google Scholar]
- 21. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 22. Messika-Zeitoun D, Thomson H, Bellamy M, Scott C, Tribouilloy C, Dearani J, Tajik AJ, Schaff H, Enriquez-Sarano M. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg 2004;128:296–302. [DOI] [PubMed] [Google Scholar]
- 23. Gammie JS, Chikwe J, Badhwar V, Thibault DP, Vemulapalli S, Thourani VH, Gillinov M, Adams DH, Rankin JS, Ghoreishi M, Wang A, Ailawadi G, Jacobs JP, Suri RM, Bolling SF, Foster NW, Quinn RW. Isolated mitral valve surgery: the Society of Thoracic Surgeons Adult cardiac surgery database analysis. Ann Thorac Surg 2018;106:716–727. [DOI] [PubMed] [Google Scholar]
- 24. Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, Park YB. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672–1678. [DOI] [PubMed] [Google Scholar]
- 25. Topilsky Y, Khanna AD, Oh JK, Nishimura RA, Enriquez-Sarano M, Jeon YB, Sundt TM, Schaff HV, Park SJ. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 2011;123:1929–1939. [DOI] [PubMed] [Google Scholar]
- 26. Yates MT, Anyanwu AC. Invited commentary. Ann Thorac Surg 2018;106:136–137. [DOI] [PubMed] [Google Scholar]
- 27. Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc 2007;39:3169–3174. [DOI] [PubMed] [Google Scholar]
- 28. Ailawadi G, Lapar DJ, Swenson BR, Siefert SA, Lau C, Kern JA, Peeler BB, Littlewood KE, Kron IL. Model for end-stage liver disease predicts mortality for tricuspid valve surgery. Ann Thorac Surg 2009;87:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N; MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 30. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Coapt I. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing with qualified researchers may be considered after submission of a proposal to Doctor Julien Dreyfus.