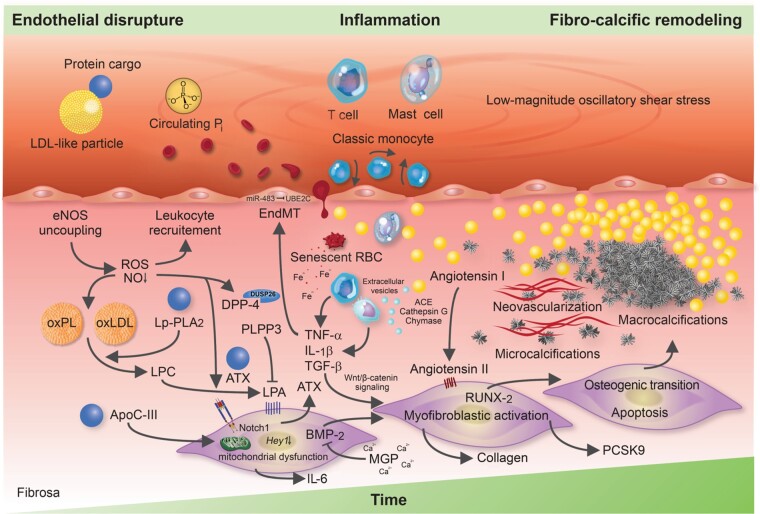
Figure 2.
Molecular and cellular mechanisms involved in calcific aortic valve disease pathogenesis. The injured endothelium covering the fibrosa fosters the uptake of immune-cells, red blood cells as well as low-density lipoprotein-like particles and their protein cargo, such as autotaxin and lipoprotein-associated phospholipase A2. Reactive oxygen species formation, enhanced by nitric oxide synthase uncoupling, aggravates the oxidative modification of lipids, promotes endothelial immune-cell trafficking and induces valvular interstitial cell apoptosis—yielding apoptotic bodies which may form additional nidi for the deposition of calcium and phosphorus crystals. While lipoprotein-associated phospholipase A2 hydrolyses the ester bond of oxidized phospholipids, autotaxin—which is secreted by valvular interstitial cells—catalyzes lysophosphatidic acid synthesis by choline group removal. Importantly, apoC-III colocalizes with calcific regions, promotes mitochondrial stress and increases interleukin-6 and bone morphogenetic protein-2 expression in human valvular interstitial cells. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinases imbalances disrupt extracellular matrix homeostasis and promote leaflet stiffening, while bone morphogenetic protein-2 drives osteogenic transition of valvular interstitial cells through increased expression of pro-osteogenic transcription factors, such as RUNX2. Infiltrated mast cells release chymase which facilitates angiotensin II synthesis, thereby promoting valvular interstitial cell-mediated collagen production and thus stiffening of aortic valve leaflets—a potent promoter of osteogenic valvular interstitial cell differentiation. Neovascularization, fuelled by vascular endothelial growth factor secretion, exacerbates immune-cell recruitment and cytokine secretion, which in turn boosts the fibro-calcific response.