Abstract
Mutations in CCAAT enhancer binding protein A gene (CEBPA) are one of the common genetic alterations in acute myeloid leukemia (AML). Recently, the emergence of new evidence makes it necessary to reconsider the subsets and treatment of AML patients with CEBPA mutations. This review will summarize the history of research progress of CEBPA mutations in AML, the heterogeneities of AML with CEBPA double mutations (CEBPAdm), and two special subtypes of CEBPA mutated AML. We will discuss the treatment of AML with CEBPA mutations as well, and finally propose a new algorithm for the treatment of these patients, including both familial and sporadic CEBPA mutated AML patients. This review may be beneficial for further investigation and optimizing clinical management of AML patients with CEBPA mutations.
Keywords: acute myeloid leukemia, CEBPA mutations, subsets, prognosis, treatment
Introduction
CCAAT enhancer binding protein alpha (CEBPα) is a crucial transcription factor for the differentiation of granulocytes, which also plays a critical role in regulating glucose metabolism (1). CEBPα is encoded by the CEBPA gene located in chromosome 19 of human, which contains two transactivation domains (TAD) in the N-terminal and one basic leucine zipper region (bZIP) in the C-terminal. CEBPA mutations are one of the most frequent genetic lesions in patients with acute myeloid leukemia (AML). Although mutations of CEBPA gene can occur across the whole gene, they cluster in two main hotspots: N-terminal frame-shift insertions/deletions and/or C-terminal in-frame insertions/deletions. Mutations in the N-terminal result in the production of a truncated protein p30, which has a dominant negative effect over the full-length p42 protein, while mutations in the C-terminal will disrupt the binding of CEBPα to DNA or dimerization (2). CEBPA mutations include those locate in one terminal (CEBPA single mutation; CEBPAsm) and those that occur in both N- and C-terminals (CEBPA double mutations; CEBPAdm). Although CEBPA mutations are widely investigated in numerous studies and several reviews have already been published to discuss their molecular mechanisms and clinical relevance (3–7), newly emerging evidence makes it necessary to reconsider the pathogenesis, subsets, and treatment choice of AML with CEBPA mutations. The aim of this perspective review is to summarize the latest findings in this field and propose a new treatment algorithm based on the available evidence.
Key Research Progress of CEBPA Mutations in AML
The frequency of CEBPA mutations in AML is 6.86%–20.33%, and a higher incidence rate is observed in AML patients from Asia compared to that in Western countries. Moreover, the frequencies of CEBPAsm and CEBPAdm are similar in AML patients from Caucasian populations, but more patients present with CEBPAdm in Asian populations (2, 6, 8–13) (Figure 1).
Figure 1.
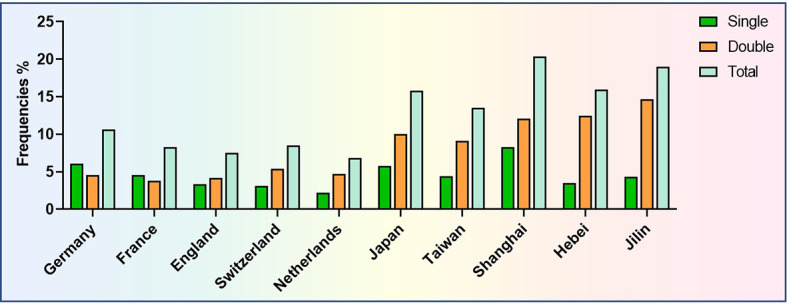
Frequencies of CEBPA mutations in AML patients from different countries or different regions of China.
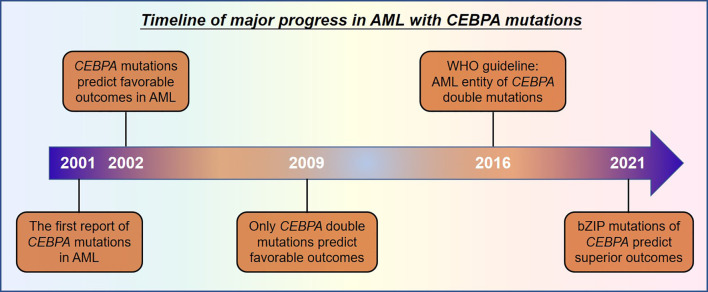
The first study was published in 2001, reporting that CEBPA mutations were identified in 10 of 137 patients with AML, which was also the first report showing CEBPA mutations in human neoplasia (14). In the following year, the prognostic significance of CEBPA mutations was retrospectively analyzed in 135 AML (non-M3) patients. Fifteen patients were found harboring CEBPA mutations, which was demonstrated to be an independent favorable prognostic factor for long-term outcomes (15). In 2009, the prognostic significance of CEBPAsm and CEBPAdm was put forward by investigators from the Netherlands (13). Only patients with CEBPAdm show a unique gene expression profile and favorable event-free survival (EFS) and overall survival (OS). However, both gene expression signature and outcomes were similar between patients with CEBPAsm and wild-type CEBPA (13). Subsequently, a series of studies confirmed the favorable prognosis of AML with CEBPAdm, both in the whole patient cohort and those with normal karyotype (9, 10, 16, 17). Thus, AML with CEBPAdm is recognized as a definite entity in “The 2016 revision to the World Health Organization classification of myeloid neoplasm and acute leukemia”, given its distinct biological and clinical characteristics (18). However, recent studies suggest that the classification of single and double mutations may not be sufficient to reflect the biological essence and clinic significance of such kind of AML. Recently, in a retrospective study including 4,708 adult patients with AML, the results showed that patients with CEBPAdm and CEBPAsm affecting bZIP (CEBPAsmbZIP) shared similar gene expression profiles and clinical features, including younger age, higher leukocytes at diagnosis, and improved survival compared to those with CEBPAsm affecting TAD (CEBPAsmTAD). Further analysis revealed that the clinical and molecular characteristics and favorable outcomes were confined to patients carrying in-frame mutations in bZIP, regardless of single or double mutations, in terms of superior complete remission (CR) rates and long-term survival (19). The favorable prognosis of CEBPAsmbZIP was also observed in another independent patient cohort of 1,028 AML patients, and presence of CEBPAsmbZIP was a strong indicator of a higher chance to achieve CR, better survival, and lower risk of relapse (20). These studies may challenge the current concept of CEBPA mutations in diagnosis and treatment of patients with AML. New subsets of AML with bZIP or non-bZIP mutations of CEBPA may be recognized rather than single and double mutations. Moreover, the prognostic and therapeutic implications of AML with CEBPAsmbZIP may be similar to those with CEBPAdm. The major research progress of CEBPA mutated AML in the last two decades was summarized in Figure 2.
Figure 2.
Major research progress of AML patients with CEBPA mutations.
Heterogeneities of AML With CEBPAdm
Although AML patients with CEBPAdm show favorable outcomes, relapse after treatment is inevitable in many patients. Therefore, the heterogeneities of AML with CEBPAdm have been noticed and discussed by our team and other investigators (2–6). Here, we divide these heterogeneities into two major categories, namely, genetic and treatment response heterogeneities.
Genetic Heterogeneity
Mutations in transcription factor GATA2 were one of the most common molecular alterations in AML patients with CEBPAdm. In the preliminary study, whole exome sequencing was performed with five patients with CEBPAdm and GATA2 zinc finger 1 (ZF1) mutations were identified in two patients (21). The authors also found that the frequency of GATA2 ZF1 mutations was 39.4% in AML patients with CEBPAdm, which tended to be a favorable indicator (21). Thereafter, several studies evaluated the prognostic significance of GATA2 mutations in patients with CEBPAdm (22–26) (summarized in Table 1). However, controversial results were found in those reports. Notably, high co-occurrence of other genetic mutations, such as FLT3-ITD in patients with wide-type GATA2, may produce unfavorable impact on the survival compared to those with mutated GATA2.
Table 1.
Frequencies and clinical significance of GATA2 mutations in AML with CEBPAdm.
| Studies | Frequencies | ED | CR | EFS | OS |
|---|---|---|---|---|---|
| Fasan et al. (22) | 18.3% (9/98) | NA | NA | Fav tendency | Fav |
| Grossmann et al. (24) | 21.0% (20/95) | NA | NA | Fav tendency | Fav |
| Green et al. (23) | 27.3% (15/55) | NS | NS | NA | NS |
| Marceau-Renaut et al. (25) | 28.7% (25/87) | NA | NA | NA | NS |
| Theis et al. (26) | 31.9% (36/113) | NS | NS | NS | NS |
ED, early death; CR, complete remission; EFS, event-free survival; OS, overall survival; Fav, favorable; NA, not available; NS, not significant.
CSF3R is the receptor of granulocyte-colony stimulating factor (G-CSF), which functions through activation of the JAK/STAT signaling pathway. High occurrence of CSF3R mutations in AML patients with CEBPAdm was first identified by RNA-sequencing in four of 14 patients, and all were T681I mutations (27). Meanwhile, high-frequency recurrent mutations in CSF3R were found with TARGET dataset of pediatric AML patients with CEBPA mutations (28). For the first time, we demonstrated that CSF3R mutations were associated with inferior survival in patients with AML with CEBPAdm (5). Interestingly, CSF3R mutations were included in two recent studies as a parameter for prognostic nomograph models (29, 30). Thus, a high degree of overlap between CSF3R and CEBPA mutations may facilitate an in-depth understanding of the role of CSF3R in the pathogenesis and prognosis of AML patients with CEBPAdm, and development of new targeted therapy, which will be discussed in a subsequent section.
Other mutated genes, such as TET2 and WT1, were reported to be negative indicators for the prognoses of AML patients with CEBPAdm (2, 3, 6, 24). Further studies are still needed to confirm these conclusions due to limited studies and relatively small numbers of patients with mutations. One effective way to solve the issue of small patient size is to combine patients with mutations according to gene family or pathways. Mutations of tyrosine kinase genes, including FLT3, CSF3R, KIT, and JAK3, confer adverse prognosis (31). Two genetic subgroups were defined by the presence (positive; pos) or absence (negative; neg) of mutations in chromatin/DNA modifiers (C), cohesin complex (C), and splicing (S) genes: CCSpos and CCSneg, respectively. Only patients with CEBPAdm with CCSneg had distinct genetic and clinical features and favorable outcomes compared to those with CEBPAsm (3). Interestingly, most patients (20/25, 80%) in the CCSpos group were defined by TET2 mutations in this study, which may reflect the unfavorable impact of TET2 mutations on the survival of AML with CEBPAdm.
Treatment Response Heterogeneity
Although CR rate after induction chemotherapy is very high in patients with CEBPAdm, a substantial proportion of the patients (30%–50%) will relapse consolidated with chemotherapeutic agents only (2, 32, 33), which suggests the heterogeneity of treatment responses of these patients. Measurable residual disease (MRD) status is a very important indicator for treatment responses and prognosis in patients with AML, which is also a potential biomarker for prognostic restratification of AML with CEBPAdm. In the preliminary single-center study, patients with CEBPAdm were divided into MRD high-risk (positive after two consolidation cycles and/or negative status loss at any time) and low-risk (persistent negative) groups based on MRD status during consolidation therapy (33). As expected, MRD risk groups were the only independent risk factor for relapse and long-term survival in multivariate analysis (33). Subsequently, we conducted a multicenter retrospective study that also confirmed the previous findings that only MRD low risk associated with low recurrence rate and superior outcomes in multivariate analysis (unpublished). Therefore, MDR status may be a potential indicator to be considered for treatment choice in patients with CEBPAdm. However, it should be noted that these two categories of heterogeneities may not be separated absolutely, because we notice that patients with high-risk genetic mutations, such as mutated CSF3R, had a significantly higher rate of positive MRD than those with wide-type CSF3R after consolidation therapy (82.0% vs. 56.25%, respectively).
Specific Subtypes of AML With CEBPA Mutations
Pediatric Patients With CEBPA Mutations
AML in adults and children may show different biological behaviors, treatment responses, or prognoses. In 2005, the first study reported that the frequency of CEBPA mutations was 6.19% (7/113) in pediatric patients with AML, including two with single and five with double mutations. Four of the seven patients had cooperating mutations with FLT3-ITD or NRAS mutations (34). The prevalence and prognostic significance of CEBPA mutations were evaluated in 847 children with AML from 3 consecutive clinical trials. CEBPA mutations were detected in 38 patients (4.49%), with 31 cases harboring double mutations. Patients with CEBPA mutations had significantly improved EFS and OS, and lower cumulative incidence rate of relapse compared to those with wide-type CEBPA (35). Single (n = 7) or double (n = 31) mutations had no significant impact on the prognosis of these patients (35), which may be due to the small size of patients in each arm. In another study from Japan, a high frequency of CEBPA mutations (14.92%, 47/315) was observed, and CEBPAdm is an independent favorable prognostic risk factor in pediatric AML patients in multivariate analysis in the total patient cohort (36). Hence, the favorable prognostic significance of CEBPA mutations could also be confirmed in pediatric patients with AML.
Familial AML With CEBPA Mutations
As early as 1978, a large familial aggregation leukemia was reported, and 13 individuals over four generations of a family comprising 293 members were diagnosed (37). After screening of genetic markers, karyotypes, and virus infections, the authors postulated that such aggregation of leukemia cases likely resulted from undefined genetic, probably polygenic, predisposition, in association with the activity of leukemogenic factors (37). However, the riddle was solved 30 years later. In 2010, a report based on one member of this family (III-45) was diagnosed as AML carrying a single heterozygous base pair deletion of the N-terminal (c.68delC) in somatic sample and a probable acquired three-base pair duplication (c.937_939dupAAG) in the C-terminal of CEBPA in a proportion of peripheral blood cells, indicating familial AML with CEBPA mutations (38). Small cases of familial AML with CEBPA mutations were also reported by other studies (23, 39). In 2015, the first study exploring the disease evolution and outcomes of familial AML with germline CEBPA mutations was reported, and 24 members from 10 CEBPA-mutated families were enrolled (40). Germline CEBPA mutations clustered within the N-terminal and acquired mutations preferentially targeting the C-terminal in diagnostic leukemia samples. AML patients with germline CEBPA mutations showed absence of diagnostic CEBPA mutations in relapse (40) and younger age than those with sporadic CEBPA mutations (41). Furthermore, patients with familial CEBPA mutations showed a favorable long-term outcome with 10-year OS of 67% (40). Although familial AML with CEBPA mutations is a rare disease, these studies discovered the unique biological behaviors and favorable prognosis of these patients.
Treatment Strategies for Patients With CEBPA Mutations
High CR rates of de novo (~90%) and relapsed (~80%) AML patients with CEBPAdm induced by chemotherapy indicate that this subtype of AML is highly sensitive to chemotherapeutic agents (42). Furthermore, with the insight into the pathogenesis and clinical features of CEBPA mutated AML in recent years, therefore, it is necessary to reconsider the treatment choice for these patients. A comparison between hematopoietic stem cell transplantation (HSCT) and chemotherapy was performed with 124 patients with CEBPAdm in CR1. Thirty-two patients were treated with allogeneic HSCT (allo-HSCT), 20 with autologous HSCT (auto-HSCT), and the remaining 72 received chemotherapy. Although patients consolidated with chemotherapy showed significantly higher relapse rates compared to those in both auto-HSCT and allo-HSCT groups, such advantage did not translate into survival benefit for HSCT. Furthermore, there is no significant difference between patients in auto-HSCT and allo-HSCT groups in terms of relapse-free survival and OS (32). Relapsed patients still have a favorable outcome after reinduction followed by allo-HSCT with a 3-year OS of 46% (32). Allo-HSCT and chemotherapy were also compared in AML patients with CEBPAdm in other studies. Allo-HSCT (n = 25) resulted in significantly lower incidence rate of relapse than chemotherapy (n = 24), but OS was similar between those two groups (43). Another study favored chemotherapy, not allo-HSCT, for patients with CEBPAdm (44). In a recent study, CEBPAdm AML patients were divided into low- and high-risk groups according to a nomograph model that was constructed with high white blood cell counts, DNA methylation related gene, CSF3R, and KMT2A mutations. Allo-HSCT was superior to chemotherapy and was only observed in high-risk, but not low-risk subgroups (29). Collectively, these results suggest that the majority of studies showed that allo-HSCT was not superior to chemotherapy or auto-HSCT in AML with CEBPAdm. Nevertheless, certain AML patients with CEBPAdm may benefit from allo-HSCT, but further study is needed to explore and validate.
With the emerging research advances, other potential targets that are reported in AML with CEBPAdm may be used for treatment. AML with CEBPAdm showed a low genetic expression signature, and reactivation of these low expressed genes promoted granulocytic differentiation of primary samples by histone deacetylase inhibitors that may be a candidate for treatment (45). High frequency of CSF3R mutations was discovered in AML with CEBPAdm, which was sensitive to JAK inhibition; furthermore, AML patients with CEBPAdm with special gene expression prolife without CSF3R mutations were uniformly sensitive to JAK inhibitors as well, which suggests the possibility of using JAK inhibitors in those patients (27). In addition, a combination of inhibitors of JAK signaling pathway and lysine-specific demethylase 1 is effectively capable of controlling the growth of CSF3R/CEBPA mutant leukemia in vivo (46). The interaction between MLL histone-methyltransferase complex with CEBPα p30 plays a critical role in leukemogenesis of CEBPA mutated AML, while MLL inhibition impairs proliferation and restores myeloid differentiation in AML cells with CEBPA mutations (47). As both histone deacetylase inhibitor Chidamide and JAK inhibitor Ruxolitinib have been used in clinic, integration of these inhibitors with chemotherapy or HSCT may possibly improve the prognosis of AML with CEBPA mutations.
Conclusion and Future Directions
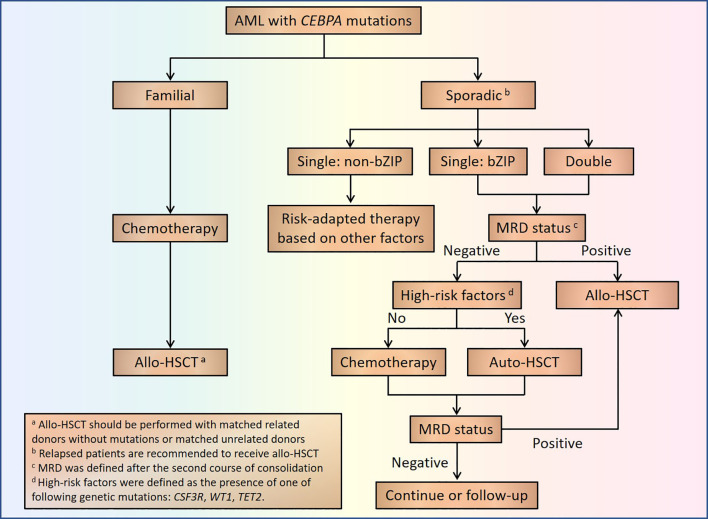
From what was discussed above, we could see that AML patients with CEBPAdm are sensitive to chemotherapy, which suggests a critical role of chemotherapy and auto-HSCT in the treatment of those patients. Although some genetic mutations are associated with high risk of relapse (CSF3R, WT1, and TET2; high-risk factors) in AML with CEBPAdm, the total frequency of those mutations is higher than the recurrence rate of CEBPAdm patients consolidated with auto-HSCT, which indicates that patients with those high-risk factors may also benefit from auto-HSCT. Furthermore, as the majority of patients with CEBPAdm carry mutations in bZIP, it will result in limited significance to divide CEBPAdm into those with or without bZIP mutations. However, recent research indicates that CEBPAsm located in bZIP showed similar clinical features and prognosis to those with CEBPAdm. Therefore, we propose that AML patients with sporadic CEBPA mutations should be divided into CEBPAsmnon-bZIP, CEBPAsmbZIP, and CEBPAdm for further treatment. For those with CEBPAsmbZIP and CEBPAdm, they should be treated according to MRD status and genetic high-risk factors for choosing chemotherapy, auto-HSCT, or allo-HSCT as we presented in Figure 3. Optimization of prognostic evaluation and treatment choice for AML patients with CEBPA mutations by MRD status during treatment here may suggest that an integrated prognostic system should be established with both pre-treatment (cytogenetic and genetic alterations) and post-treatment (MRD status) parameters, in order to direct choosing treatment strategies post remission. As to those with familial AML with CEBPA mutations, favorable outcomes could be achieved by chemotherapy, and those with refractory or relapse disease should receive allo-HSCT to eliminate the germline mutations with related donors without mutations or unrelated donors (Figure 3).
Figure 3.
Treatment flowchart of AML patients with CEBPA mutations.
More beneficial evidence that CEBPA bZIP mutations may define a subset of AML is still anticipated, especially in the settings of different populations or treatment plans. Some investigators suggested the classification of CEBPA mutated AML as CEBPA with in-frame bZIP mutations and those without. However, two points must be mentioned. First, the frequency of frame-shift bZIP mutations in CEBPAdm is very low in AML in some patient cohorts; it is only 4.38% (6/137) in patients from our center. Second, a comparison between in-frame and frame-shift bZIP mutations of CEBPA is still needed. Furthermore, whether such phenomenon could be observed in pediatric AML patients needs further exploration. Although AML with CEBPAdm is sensitive to chemotherapy, evidence of auto-HSCT is limited, which may be helpful to prevent disease relapse in some patients because auto-HSCT is more intensive than chemotherapy alone. Finally, with the discovery of new potential targets or development and application of new drugs in the treatment of those patients, the prognoses of CEBPA mutated AML may be further improved, which may challenge the diagnosis and treatment dogma of the current concept.
Author Contributions
LS wrote the manuscript. LS, Y-YS, Z-YL, and S-JG collected, analyzed, and summarized the data. LS, Y-YS, Z-YL, and S-JG conceptualized this review. LS and S-JG revised the review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from NSFC (81900174) and the Clinical Research Foundation of First Hospital of Jilin University (No. LCFYJJ2017005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Nerlov C. C/EBPalpha Mutations in Acute Myeloid Leukaemias. Nat Rev Cancer (2004) 4:394–400. doi: 10.1038/nrc1363 [DOI] [PubMed] [Google Scholar]
- 2. Su L, Tan Y, Lin H, Liu X, Yu L, Yang Y, et al. Mutational Spectrum of Acute Myeloid Leukemia Patients With Double CEBPA Mutations Based on Next-Generation Sequencing and its Prognostic Significance. Oncotarget (2018) 9:24970–9. doi: 10.18632/oncotarget.23873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konstandin NP, Pastore F, Herold T, Dufour A, Rothenberg-Thurley M, Hinrichsen T, et al. Genetic Heterogeneity of Cytogenetically Normal AML With Mutations of CEBPA. Blood Adv (2018) 2:2724–31. doi: 10.1182/bloodadvances.2018016840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nie Y, Su L, Li W, Gao S. Novel Insights of Acute Myeloid Leukemia With CEBPA Deregulation: Heterogeneity Dissection and Re-Stratification. Crit Rev Oncol Hematol (2021) 163:103379. doi: 10.1016/j.critrevonc.2021.103379 [DOI] [PubMed] [Google Scholar]
- 5. Su L, Gao S, Tan Y, Lin H, Liu X, Liu S, et al. CSF3R Mutations Were Associated With an Unfavorable Prognosis in Patients With Acute Myeloid Leukemia With CEBPA Double Mutations. Ann Hematol (2019) 98:1641–6. doi: 10.1007/s00277-019-03699-7 [DOI] [PubMed] [Google Scholar]
- 6. Tien FM, Hou HA, Tang JL, Kuo YY, Chen CY, Tsai CH, et al. Concomitant WT1 Mutations Predict Poor Prognosis in Acute Myeloid Leukemia Patients With Double Mutant CEBPA. Haematologica (2018) 103:e510–3. doi: 10.3324/haematol.2018.189043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilhelmson AS, Porse BT. CCAAT Enhancer Binding Protein Alpha (CEBPA) Biallelic Acute Myeloid Leukaemia: Cooperating Lesions, Molecular Mechanisms and Clinical Relevance. Br J Haematol (2020) 190:495–507. doi: 10.1111/bjh.16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fasan A, Haferlach C, Alpermann T, Jeromin S, Grossmann V, Eder C, et al. The Role of Different Genetic Subtypes of CEBPA Mutated AML. Leukemia (2014) 28:794–803. doi: 10.1038/leu.2013.273 [DOI] [PubMed] [Google Scholar]
- 9. Green CL, Koo KK, Hills RK, Burnett AK, Linch DC, Gale RE. Prognostic Significance of CEBPA Mutations in a Large Cohort of Younger Adult Patients With Acute Myeloid Leukemia: Impact of Double CEBPA Mutations and the Interaction With FLT3 and NPM1 Mutations. J Clin Oncol (2010) 28:2739–47. doi: 10.1200/JCO.2009.26.2501 [DOI] [PubMed] [Google Scholar]
- 10. Pabst T, Eyholzer M, Fos J, Mueller BU. Heterogeneity Within AML With CEBPA Mutations; Only CEBPA Double Mutations, But Not Single CEBPA Mutations Are Associated With Favourable Prognosis. Br J Cancer (2009) 100:1343–6. doi: 10.1038/sj.bjc.6604977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Renneville A, Boissel N, Gachard N, Naguib D, Bastard C, de Botton S, et al. The Favorable Impact of CEBPA Mutations in Patients With Acute Myeloid Leukemia is Only Observed in the Absence of Associated Cytogenetic Abnormalities and FLT3 Internal Duplication. Blood (2009) 113:5090–3. doi: 10.1182/blood-2008-12-194704 [DOI] [PubMed] [Google Scholar]
- 12. Shen Y, Zhu YM, Fan X, Shi JY, Wang QR, Yan XJ, et al. Gene Mutation Patterns and Their Prognostic Impact in a Cohort of 1185 Patients With Acute Myeloid Leukemia. Blood (2011) 118:5593–603. doi: 10.1182/blood-2011-03-343988 [DOI] [PubMed] [Google Scholar]
- 13. Wouters BJ, Löwenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA Mutations, But Not Single CEBPA Mutations, Define a Subgroup of Acute Myeloid Leukemia With a Distinctive Gene Expression Profile That Is Uniquely Associated With a Favorable Outcome. Blood (2009) 113:3088–91. doi: 10.1182/blood-2008-09-179895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-Negative Mutations of CEBPA, Encoding CCAAT/enhancer Binding Protein-Alpha (C/EBPalpha), in Acute Myeloid Leukemia. Nat Genet (2001) 27:263–70. doi: 10.1038/85820 [DOI] [PubMed] [Google Scholar]
- 15. Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, et al. Favorable Prognostic Significance of CEBPA Mutations in Patients With De Novo Acute Myeloid Leukemia: A Study From the Acute Leukemia French Association (ALFA). Blood (2002) 100:2717–23. doi: 10.1182/blood-2002-03-0990 [DOI] [PubMed] [Google Scholar]
- 16. Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, et al. Acute Myeloid Leukemia With Biallelic CEBPA Gene Mutations and Normal Karyotype Represents a Distinct Genetic Entity Associated With a Favorable Clinical Outcome. J Clin Oncol (2010) 28:570–7. doi: 10.1200/JCO.2008.21.6010 [DOI] [PubMed] [Google Scholar]
- 17. Taskesen E, Bullinger L, Corbacioglu A, Sanders MA, Erpelinck CA, Wouters BJ, et al. Prognostic Impact, Concurrent Genetic Mutations, and Gene Expression Features of AML With CEBPA Mutations in a Cohort of 1182 Cytogenetically Normal AML Patients: Further Evidence for CEBPA Double Mutant AML as a Distinctive Disease Entity. Blood (2011) 117:2469–75. doi: 10.1182/blood-2010-09-307280 [DOI] [PubMed] [Google Scholar]
- 18. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 19. Taube F, Georgi JA, Kramer M, Stasik S, Middeke JM, Röllig C, et al. CEBPA Mutations in 4708 Patients With Acute Myeloid Leukemia - Differential Impact of bZIP and TAD Mutations on Outcome. Blood (2022) 139:87–103. doi: 10.1182/blood.2020009680 [DOI] [PubMed] [Google Scholar]
- 20. Wakita S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic Impact of CEBPA bZIP Domain Mutation in Acute Myeloid Leukemia. Blood Adv (2021) 6:238–47. doi: 10.1182/bloodadvances.2021004292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greif PA, Dufour A, Konstandin NP, Ksienzyk B, Zellmeier E, Tizazu B, et al. GATA2 Zinc Finger 1 Mutations Associated With Biallelic CEBPA Mutations Define a Unique Genetic Entity of Acute Myeloid Leukemia. Blood (2012) 120:395–403. doi: 10.1182/blood-2012-01-403220 [DOI] [PubMed] [Google Scholar]
- 22. Fasan A, Eder C, Haferlach C, Grossmann V, Kohlmann A, Dicker F, et al. GATA2 Mutations Are Frequent in Intermediate-Risk Karyotype AML With Biallelic CEBPA Mutations and Are Associated With Favorable Prognosis. Leukemia (2013) 27:482–5. doi: 10.1038/leu.2012.174 [DOI] [PubMed] [Google Scholar]
- 23. Green CL, Tawana K, Hills RK, Bödör C, Fitzgibbon J, Inglott S, et al. GATA2 Mutations in Sporadic and Familial Acute Myeloid Leukaemia Patients With CEBPA Mutations. Br J Haematol (2013) 161:701–5. doi: 10.1111/bjh.12317 [DOI] [PubMed] [Google Scholar]
- 24. Grossmann V, Haferlach C, Nadarajah N, Fasan A, Weissmann S, Roller A, et al. CEBPA Double-Mutated Acute Myeloid Leukaemia Harbours Concomitant Molecular Mutations in 76·8% of Cases With TET2 and GATA2 Alterations Impacting Prognosis. Br J Haematol (2013) 161:649–58. doi: 10.1111/bjh.12565 [DOI] [PubMed] [Google Scholar]
- 25. Marceau-Renaut A, Guihard S, Castaigne S, Dombret H, Preudhomme C, Cheok M. Classification of CEBPA Mutated Acute Myeloid Leukemia by GATA2 Mutations. Am J Hematol (2015) 90:E93–94. doi: 10.1002/ajh.23949 [DOI] [PubMed] [Google Scholar]
- 26. Theis F, Corbacioglu A, Gaidzik VI, Paschka P, Weber D, Bullinger L, et al. Clinical Impact of GATA2 Mutations in Acute Myeloid Leukemia Patients Harboring CEBPA Mutations: A Study of the AML Study Group. Leukemia (2016) 30:2248–50. doi: 10.1038/leu.2016.185 [DOI] [PubMed] [Google Scholar]
- 27. Lavallée VP, Krosl J, Lemieux S, Boucher G, Gendron P, Pabst C, et al. Chemo-Genomic Interrogation of CEBPA Mutated AML Reveals Recurrent CSF3R Mutations and Subgroup Sensitivity to JAK Inhibitors. Blood (2016) 127:3054–61. doi: 10.1182/blood-2016-03-705053 [DOI] [PubMed] [Google Scholar]
- 28. Maxson JE, Ries RE, Wang YC, Gerbing RB, Kolb EA, Thompson SL, et al. CSF3R Mutations Have a High Degree of Overlap With CEBPA Mutations in Pediatric AML. Blood (2016) 127:3094–8. doi: 10.1182/blood-2016-04-709899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu LX, Jiang H, Chang YJ, Zhou YL, Wang J, Wang ZL, et al. Risk Stratification of Cytogenetically Normal Acute Myeloid Leukemia With Biallelic CEBPA Mutations Based on a Multi-Gene Panel and Nomogram Model. Front Oncol (2021) 11:706935. doi: 10.3389/fonc.2021.706935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu X, Cai W, Cai P, Zhang L, Yao H, Zhang T, et al. Prognostic Nomogram for Acute Myeloid Leukemia Patients With Biallelic CEBPA Mutations. Front Oncol (2021) 11:628248. doi: 10.3389/fonc.2021.628248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Wang F, Chen X, Zhang Y, Wang M, Liu H, et al. Companion Gene Mutations and Their Clinical Significance in AML With Double Mutant CEBPA. Cancer Gene Ther (2020) 27:599–606. doi: 10.1038/s41417-019-0133-7 [DOI] [PubMed] [Google Scholar]
- 32. Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, et al. The Value of Allogeneic and Autologous Hematopoietic Stem Cell Transplantation in Prognostically Favorable Acute Myeloid Leukemia With Double Mutant CEBPA. Blood (2013) 122:1576–82. doi: 10.1182/blood-2013-05-503847 [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Lu R, Wu Y, Jia J, Gong L, Liu X, et al. Detection of Measurable Residual Disease may Better Predict Outcomes Than Mutations Based on Next-Generation Sequencing in Acute Myeloid Leukaemia With Biallelic Mutations of CEBPA. Br J Haematol (2020) 190:533–44. doi: 10.1111/bjh.16535 [DOI] [PubMed] [Google Scholar]
- 34. Liang DC, Shih LY, Huang CF, Hung IJ, Yang CP, Liu HC, et al. CEBPalpha Mutations in Childhood Acute Myeloid Leukemia. Leukemia (2005) 19:410–4. doi: 10.1038/sj.leu.2403608 [DOI] [PubMed] [Google Scholar]
- 35. Ho PA, Alonzo TA, Gerbing RB, Pollard J, Stirewalt DL, Hurwitz C, et al. Prevalence and Prognostic Implications of CEBPA Mutations in Pediatric Acute Myeloid Leukemia (AML): A Report From the Children's Oncology Group. Blood (2009) 113:6558–66. doi: 10.1182/blood-2008-10-184747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuo H, Kajihara M, Tomizawa D, Watanabe T, Saito AM, Fujimoto J, et al. Prognostic Implications of CEBPA Mutations in Pediatric Acute Myeloid Leukemia: A Report From the Japanese Pediatric Leukemia/Lymphoma Study Group. Blood Cancer J (2014) 4:e226. doi: 10.1038/bcj.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunz FW, Gunz JP, Vincent PC, Bergin M, Johnson FL, Bashir H, et al. Thirteen Cases of Leukemia in a Family. J Natl Cancer Inst (1978) 60:1243–50. doi: 10.1093/jnci/60.6.1243 [DOI] [PubMed] [Google Scholar]
- 38. Carmichael CL, Wilkins EJ, Bengtsson H, Horwitz MS, Speed TP, Vincent PC, et al. Poor Prognosis in Familial Acute Myeloid Leukaemia With Combined Biallelic CEBPA Mutations and Downstream Events Affecting the ATM, FLT3 and CDX2 Genes. Br J Haematol (2010) 150:382–5. doi: 10.1111/j.1365-2141.2010.08204.x [DOI] [PubMed] [Google Scholar]
- 39. Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in Familial Acute Myeloid Leukemia. N Engl J Med (2004) 351:2403–7. doi: 10.1056/NEJMoa041331 [DOI] [PubMed] [Google Scholar]
- 40. Tawana K, Wang J, Renneville A, Bödör C, Hills R, Loveday C, et al. Disease Evolution and Outcomes in Familial AML With Germline CEBPA Mutations. Blood (2015) 126:1214–23. doi: 10.1182/blood-2015-05-647172 [DOI] [PubMed] [Google Scholar]
- 41. Shih LY, Liang DC, Huang CF, Wu JH, Lin TL, Wang PN, et al. AML Patients With CEBPalpha Mutations Mostly Retain Identical Mutant Patterns But Frequently Change in Allelic Distribution at Relapse: A Comparative Analysis on Paired Diagnosis and Relapse Samples. Leukemia (2006) 20:604–9. doi: 10.1038/sj.leu.2404124 [DOI] [PubMed] [Google Scholar]
- 42. Kurosawa S, Yamaguchi H, Yamaguchi T, Fukunaga K, Yui S, Kanamori H, et al. The Prognostic Impact of FLT3-ITD, NPM1 and CEBPa in Cytogenetically Intermediate-Risk AML After First Relapse. Int J Hematol (2020) 112:200–9. doi: 10.1007/s12185-020-02894-x [DOI] [PubMed] [Google Scholar]
- 43. Ahn JS, Kim JY, Kim HJ, Kim YK, Lee SS, Jung SH, et al. Normal Karyotype Acute Myeloid Leukemia Patients With CEBPA Double Mutation Have a Favorable Prognosis But No Survival Benefit From Allogeneic Stem Cell Transplant. Ann Hematol (2016) 95:301–10. doi: 10.1007/s00277-015-2540-7 [DOI] [PubMed] [Google Scholar]
- 44. Kurosawa S, Yamaguchi H, Yamaguchi T, Fukunaga K, Yui S, Wakita S, et al. Decision Analysis of Postremission Therapy in Cytogenetically Intermediate-Risk Acute Myeloid Leukemia: The Impact of FLT3 Internal Tandem Duplication, Nucleophosmin, and CCAAT/Enhancer Binding Protein Alpha. Biol Blood Marrow Transplant (2016) 22:1125–32. doi: 10.1016/j.bbmt.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 45. Liss A, Ooi CH, Zjablovskaja P, Benoukraf T, Radomska HS, Ju C, et al. The Gene Signature in CCAAT-Enhancer-Binding Protein α Dysfunctional Acute Myeloid Leukemia Predicts Responsiveness to Histone Deacetylase Inhibitors. Haematologica (2014) 99:697–705. doi: 10.3324/haematol.2013.093278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braun TP, Coblentz C, Smith BM, Coleman DJ, Schonrock Z, Carratt SA, et al. Combined Inhibition of JAK/STAT Pathway and Lysine-Specific Demethylase 1 as a Therapeutic Strategy in CSF3R/CEBPA Mutant Acute Myeloid Leukemia. Proc Natl Acad Sci USA (2020) 117:13670–9. doi: 10.1073/pnas.1918307117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmidt L, Heyes E, Scheiblecker L, Eder T, Volpe G, Frampton J, et al. CEBPA-Mutated Leukemia Is Sensitive to Genetic and Pharmacological Targeting of the MLL1 Complex. Leukemia (2019) 33:1608–19. doi: 10.1038/s41375-019-0382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]